Abstract
Introduction
Previous studies on anticoagulation treatment trends have mostly focused on hospitalized patients. This study aimed to clarify the treatment status of patients with venous thromboembolism (VTE) in Japan from 2011 to 2018, including outpatients, and to assess adherence with current guidelines.
Methods
Data of inpatients and outpatients who were treated for VTE were extracted from a nationwide claims database (Medical Data Vision Co., Ltd., Tokyo, Japan) and analyzed.
Results
The study included 79,330 patients with VTE; half were diagnosed during hospitalization for diseases other than VTE. The proportion of outpatient treatment increased significantly from 2015 to 2018 (Cochran–Armitage trend test, P < 0.0001), while 80% were anticoagulated in hospital after pulmonary embolism (PE) diagnosis. The proportion of patients with VTE treated as outpatients was no lower than the proportion of inpatients, even in the presence of active cancer, and there were no clear differences in anticoagulant choices. Treatment with direct oral anticoagulants (DOACs) did not always include the recommended initial intensification therapy. There was wide variation in the duration of DOAC treatment and the median duration of use was shorter than that recommended in VTE treatment guidelines.
Conclusion
While the gradual increase in VTE outpatient treatment appears to be in line with guideline recommendations, PE outpatient treatment could be further facilitated. The large proportion of patients diagnosed with VTE during hospitalization for other conditions suggests the importance of further utilizing in-hospital manuals for thrombosis prevention. The presence or absence of cancer did not appear to affect the basic treatment strategy of anticoagulation for VTE. Future studies are expected to better define the characteristics of patients who can be safely and effectively treated in an outpatient setting, and to examine whether anticoagulation for a shorter treatment period than recommended by the guidelines or DOAC therapy without initial intensification would improve patient outcomes.
Similar content being viewed by others
Why carry out this study? |
Venous thromboembolism (VTE) is a frequent and serious medical event associated with a substantial risk of adverse outcomes. |
As previous studies on anticoagulation treatment trends were mostly limited to inpatients, this study intended to examine the clinical management and demographic characteristics of patients with VTE in Japan, including outpatients, to identify treatment trends from 2011 to 2018, and to assess the adherence to current guidelines. |
What was learned from the study? |
About half of the patients diagnosed with VTE had been hospitalized for illnesses other than VTE, the proportion of outpatients treated for VTE gradually increased, and the presence of cancer did not appear to be a barrier to anticoagulant choice or to outpatient treatment. |
The duration of anticoagulation was shorter and more variable than that specified by current guidelines, and the treatment with direct oral anticoagulants (DOACs) may not always follow the suggested initial treatment strategy. |
The study findings suggest the importance of developing and further utilizing in-hospital manuals for thrombosis prevention, and future studies should examine whether the current use of DOACs, with possible deviations from guideline-recommended use and shorter anticoagulation regimens, improves patient outcomes. |
Introduction
Venous thromboembolism (VTE) comprises both deep vein thrombosis (DVT) and pulmonary embolism (PE). Reportedly, 90% of PE cases are caused by DVT in the lower limbs [1], and DVT and PE are often combined. VTE is a serious medical event associated with substantial risks of adverse outcomes, including death [2,3,4,5].
The VTE prevalence in Asian countries is lower than that in European countries and the USA [6], where VTE is the third most common cardiovascular disease [7], but has been gradually increasing in Japan [8]. The Japanese guidelines for the diagnosis, treatment, and prevention of PE and DVT [1] are based on the 2014 European Society of Cardiology guidelines [9] and the 2012 [10] and 2016 [11] American College of Chest Physicians guidelines. Recently, direct oral anticoagulant (DOAC) therapy has been recommended by guidelines and expert consensus statements of Japan, the USA, and Europe for VTE treatment and recurrence prevention [1, 12,13,14].
To date, epidemiological studies on inpatients with VTE have been conducted in Denmark [15] and Japan [16], and the frequency of hospitalization for VTE in these countries has been determined. Regarding pharmaceutical treatment, previous studies have shown that DOAC use has increased in hospitalized patients with VTE while warfarin use has decreased [16, 17]. However, these studies were mostly limited to inpatients, and the number of studies examining the epidemiology and treatment status in both inpatients and outpatients with VTE is limited. Understanding the trends in treatment settings in both inpatients and outpatients and adherence to current guidelines is vital to identifying and addressing issues in VTE treatment.
We aimed to examine the clinical management and demographic characteristics of inpatients and outpatients with VTE in current Japanese clinical practice and study the trends in the VTE treatment types preferred by medical practitioners from 2011 to 2018.
Methods
Data Source
This study analyzed data from the Medical Data Vision (MDV) database, a longitudinal database provided by Medical Data Vision Co., Ltd. (Tokyo, Japan). The database provides claims data from 399 hospitals (approximately 30 million patients, as of February 2020) using the Diagnosis Procedure Combination (DPC) system (29.6% of general hospitals and 54.1% of general beds in Japan employ the DPC system, as of March 2019). The patient data were analyzed for the period registered in this database.
Study Population
The MDV subset used for the analysis comprised all patients diagnosed with DVT or PE between 1 January 2011 and 31 December 2018. The International Classification of Diseases 10th Revision (ICD-10) codes used in this study are listed in Table S1 in the supplementary material. Eligible patients were those (1) who received a definitive VTE diagnosis and subsequent treatment; (2) aged ≥ 20 years on the date of VTE diagnosis (index date); and (3) with baseline information from a period of ≥ 3 months prior to the index date (baseline period). Key exclusion criteria were (1) a VTE diagnosis prior to MDV database entry; (2) a diagnosis of atrial fibrillation (AF) during the baseline period; (3) a diagnosis of AF within 30 days after the index date; and (4) prescription of anticoagulants for > 1 week during the baseline period.
DVT and PE were examined separately, considering the potential need for different treatments as PE has a poorer prognosis than DVT [4]. Active neoplasm/cancer is a significant risk factor for VTE [18, 19] but also a risk factor for bleeding. Thus, in patients with active cancer, safety may be more emphasized than anticoagulant efficacy. Therefore, the treatment was also examined regarding the presence or absence of active cancer.
Protection of Human Participants
This study extracted data that existed in an anonymized structured format and did not contain any personal information of patients. According to applicable legal requirements, such data are not subject to privacy laws. According to the Ethical Guidelines for Human Life Science and Medical Research in Japan, informed consent is not required for studies using unlinkable anonymized data. Therefore, obtaining informed consent from patients and institutional review board approval were not required.
Definitions
The index date was defined as the date of the first VTE diagnosis in the database (day 0). For multiple VTE diagnoses, the first diagnosis was used for the index date. Subsequent diagnoses were treated as recurrent VTE. VTE recurrence during hospitalization was disregarded. We adopted a recently validated algorithm for identifying VTE diagnoses [20].
The baseline period was from 3 to up to 6 months before the index date, and the data of this period were used as baseline information.
Patients were classified into two groups according to their diagnosis: (1) the DVT group, wherein patients were diagnosed as having DVT only; and (2) the PE group, wherein patients were diagnosed as having either only PE or both DVT and PE. The follow-up period extended from the day after the index date to the date anticoagulation therapy was completed. The patients were further classified into the following three subgroups according to their inpatient/outpatient status: (1) DVT/PE outpatients were outpatients at the time of VTE diagnosis and were subsequently treated for VTE on an outpatient basis; (2) DVT/PE inpatients 1 (hospitalized for DVT/PE treatment) were outpatients at the time of VTE diagnosis and were subsequently hospitalized for VTE treatment; and (3) DVT/PE inpatients 2 (hospitalized for purposes other than DVT/PE treatment) were already hospitalized for other reasons at the time of VTE diagnosis (the principal diagnosis of hospitalization was not DVT/PE), including patients who received initial anticoagulant treatment for VTE during hospitalization and were then discharged with continued treatment.
Patients were considered to have active cancer if patients with cancer diagnoses received anticancer treatment (drug administration, radiation therapy, or surgery) or palliative care during the baseline period or if they were newly diagnosed with any cancer during the baseline period or within 30 days after the index date. The ICD-10 codes used to confirm a cancer diagnosis are presented in Table S1 in the supplementary material.
Anticoagulants
Anticoagulants were categorized as (1) drugs used at some point throughout the analysis period and (2) drugs used at the time when anticoagulation was started. Only the following treatment options approved in Japan for the treatment of VTE and the prevention of VTE recurrence were included in this analysis: unfractionated heparin and fondaparinux as parenteral anticoagulants, DOACs (i.e., apixaban, edoxaban, and rivaroxaban), warfarin, urokinase and tissue plasminogen activator (tPA) as thrombolytic drugs, and inferior vena cava (IVC) filters. Low-molecular-weight heparin and dabigatran are not approved for VTE treatment in Japan. The types and proportions of drugs at the time of anticoagulant therapy initiation were investigated; for DOACs and warfarin, patients who were started on a single agent were investigated, and patients who received heparin and another anticoagulant, i.e., warfarin or DOAC, at the same time were included in the heparin group.
Statistical Analysis
For each calendar year from 2011 to 2018, the distribution of patients with VTE with initiated new treatments; proportions of inpatients to outpatients, of patients receiving thrombolytic therapies or undergoing IVC filter placement, and of patients treated with anticoagulation therapies; and durations of anticoagulation therapies were described. Continuous variables are presented as mean, median, and standard deviation. Categorical data are presented as numbers and percentages for dichotomous and polychotomous variables. For comparisons between groups, continuous values were subjected to the unpaired t-test and categorical data to the chi-square test.
Owing to the large sample size and the possibility of detecting small irrelevant differences, P values were calculated only for comparisons of inpatient to outpatient rates (chi-square test, 5% level of significance) and anticoagulation durations (unpaired t-test, 5% level of significance). Differences in patient characteristics between groups were assessed using standardized difference (std. diff.) scores, rather than P values, to measure the magnitude of the between-group effect size independent of the number of patients in each group. A cutoff value of 0.1 was used to judge whether the differences were negligible. Changes in the percentage of patients treated for VTE in the outpatient setting were evaluated by using the Cochran–Armitage test for trend, with a significance level of 0.05. Imputation for missing data was not performed. The statistical software SAS 9.4 was used.
Results
Study Population
Patients enrolled in the MDV database between 1 January 2011 and 31 December 2018 were included in the study. The patient selection procedure is shown in Fig. 1.
Flowchart of patient selection. The index date was defined as the date when the patient was first diagnosed with DVT or PE using ICD-10 codes. DVT deep vein thrombosis, ICD-10 International Classification of Diseases 10th Revision, PE pulmonary embolism, MDV Medical Data Vision, VTE venous thromboembolism
Temporal Trends in the Proportions of Inpatients and Outpatients
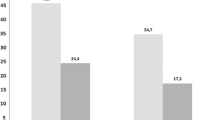
The percentages of the three categories of patients (outpatients and two types of inpatients) are shown in Fig. 2. The percentage of patients with DVT among patients with VTE (the sum of patients in the three DVT categories) increased slightly from 75.8% in 2011 to 82.4% in 2018. Inpatients hospitalized for conditions other than DVT or PE were more prevalent in both groups, constituting > 50% of patients with VTE. For both DVT and PE, the proportions of outpatient treatments increased (Fig. S1 in the supplementary material). These increases were significant between the years 2015 and 2018 in both groups (Cochran–Armitage trend test: DVT, P < 0.0001; PE, P < 0.0001); however, between 2011 and 2015, a trend toward change was not observed for DVT (Cochran–Armitage trend test: DVT, P < 0.3934; PE, P < 0.0034). Annual changes in the numbers and percentages of patients with DVT or PE are presented in Table S2 in the supplementary material. The chi-square test for the association between outpatient treatment status and the presence of cancer in VTE patients showed that outpatient treatment was significantly more common when cancer was present (Table S3 in the supplementary material).
Inpatient and outpatient proportions for DVT and PE by treatment purpose (2011–2018). The percentages of patients classified into each treatment category are shown. Outpatients These patients were outpatients at the time of DVT or PE diagnosis and were subsequently treated for DVT/PE on an outpatient basis. DVT/PE inpatients 1 These patients were outpatients at the time of VTE diagnosis and were subsequently hospitalized for VTE treatment. DVT/PE inpatients 2 These patients were already hospitalized for other reasons at the time of VTE diagnosis (the principal diagnosis of hospitalization was not DVT or PE). DVT deep vein thrombosis, PE pulmonary embolism
Differences in Patient Characteristics between Inpatients and Outpatients
Patient backgrounds are presented in Table 1 for each of the three abovementioned treatment statuses. The mean age at the index date was similar in the DVT and PE groups (std. diff. 0.047). The proportion of patients with age ≥ 80 years was > 30% in both groups. Half of the patients with VTE were diagnosed during hospitalization for other treatments. In DVT, 84% of outpatients continued treatment in the outpatient setting. Conversely, 82% of patients with PE were admitted for PE treatment. The proportion of patients with active cancer was similar in both groups (std. diff. 0.043), accounting for 27.6% and 25.7% of the DVT and PE groups, respectively. Gastrointestinal (GI) cancers were the most prevalent type in both groups.
Changes in VTE Treatment
DOAC users have outnumbered warfarin users since 2015 (Fig. 3). In 2018, DOACs were used by approximately 90% of patients with VTE who required anticoagulation therapy. The frequency of unfractionated heparin use has gradually decreased since 2015. Urokinase or tPA was administered to only a limited number of patients with DVT or PE (Fig. 4); in 2018, their frequency was approximately 1% in the DVT group and 7% in the PE group (Fig. S2 in the supplementary material). The frequency of IVC filter placement decreased to < 10% in 2018 in the DVT and PE groups (Fig. S3 in the supplementary material).
Distribution of anticoagulants used among newly treated patients with venous thromboembolism in the Medical Data Vision database. The number of patients starting anticoagulation therapy with each anticoagulant is indicated for patients with DVT (a) and patients with PE (b). The percentages of patients treated with each oral anticoagulant are indicated for patients with DVT (c) and patients with PE (d). DOAC direct oral anticoagulant, DVT deep vein thrombosis, PE pulmonary embolism
Distribution of thrombolytic therapies used for DVT or PE. The numbers of patients treated with urokinase or tPA are shown for patients with DVT (a) and patients with PE (b). The percentages of patients treated with urokinase or tPA are shown for patients with DVT (c) and patients with PE (d). DVT deep vein thrombosis, PE pulmonary embolism, tPA tissue plasminogen activator
Drug Selection and Patient Characteristics
In the majority of patients with DVT, anticoagulation was initiated with a DOAC alone. Among those patients, the proportion of edoxaban use was highest; the std. diff. for the percentage of edoxaban use relative to the overall percentages of patients with DVT who used apixaban and rivaroxaban was 0.721 and 0.780, respectively. By contrast, edoxaban use in patients with PE was limited to 13.2%. The std. diff. for the percentage of edoxaban use relative to the overall percentages of patients with PE who used apixaban and rivaroxaban was 0.120 and 0.203, respectively. For patients with PE, heparin and fondaparinux (including combinations with other anticoagulants) were the most commonly prescribed anticoagulants for initial therapy. The use of warfarin as an initial therapy without heparin lead-in was very limited in both DVT and PE. Table 2 also shows the drug use by active cancer status. Apixaban and rivaroxaban use did not differ between patients with or without cancer in both DVT and PE groups (std. diff. 0.045 and 0.049 for DVT; 0.055 and 0.032 for PE, respectively). Table S4 in the supplementary material shows the starting dose of DOACs in patients who used a DOAC at some point in their VTE treatment.
Duration of Anticoagulation Therapy
In patients who started anticoagulation with heparin or fondaparinux alone, the median duration of treatment was 6 days or less in both DVT and PE groups according to the data from 2017 to 2018 (Table 3). In outpatients, the median duration of heparin use was 1 day for both VTE and PE. When DOACs were used as initial anticoagulants, the median treatment duration was 10–30 days longer in patients with PE than in those with DVT. The median duration of treatment with DOACs ranged from 1 to 2 months for DVT and from 1.5 to 3 months for PE. This difference in duration between the two groups was significant (P < 0.0001). The first and third quartiles of DOAC use durations differed in both DVT and PE groups substantially, ranging from approximately 20 to 200 days. The treatment duration with edoxaban was generally shorter than those with the other two DOACs, especially in patients with DVT.
In patients with active cancer who started the initial treatment with a DOAC, the median duration of DOAC treatment was less than 3 months for any DOAC in patients with DVT and varied from about 2 months to over 3 months among DOACs in patients with PE. Overall in patients with VTE, the treatment duration was significantly longer for patients with active cancer (P < 0.0001).
Discussion
Patient Population
Our findings suggest that the DVT:PE ratio was approximately 4:1 in 2018, and the proportion of PE diagnoses has been gradually decreasing since 2011. A previous report from the J-ROAD-DPC [13], a disease registry for hospitalized patients with VTE in Japan, indicated that the mean patient age was 69.1 years and that the proportion of female patients was 59.6%; our present findings are similar to these results.
In this study, half of all VTEs were diagnosed during hospitalization unrelated to VTE. This may include patients who developed VTE in the high-risk environment of hospitalization or who were diagnosed with VTE during the diagnosis and treatment for other diseases. In either case, the fact that such a large number of patients were diagnosed during hospitalization suggests the need for the development or further utilization of in-hospital manuals and the need for thrombosis prevention from the perspective of medical safety.
In our study, patients with active cancer accounted for approximately 30% of all patients with VTE, and approximately 45% had GI cancer (gastrointestinal tract, gallbladder, liver, or pancreas). GI cancers are the most prevalent cancer forms in Japan (29.8% of all cancers) [21], which could explain why GI cancer was frequently observed in patients with VTE. The comorbidity rate of pathological conditions other than cancer such as congestive heart failure, surgery, trauma, and lower-extremity fracture was relatively high; these conditions are listed as risk factors in VTE guidelines [1].
Inpatient and Outpatient Treatment
We found that the percentage of patients treated in outpatient settings has increased since 2015, likely due to the expanded use of all three DOACs for VTE treatment in 2015. Additionally, more than 80% of patients diagnosed with DVT in an outpatient setting are treated without hospitalization. According to Japanese treatment guidelines [1], outpatient treatment is recommended if patients with DVT do not have any PE signs and fulfill various other criteria. The current real-world practice regarding DVT management appears to follow these guidelines [1]. Conversely, more than 80% of patients with PE diagnosed in outpatient settings were hospitalized for treatment with anticoagulants immediately after diagnosis. This may be due to the urgency and importance of treating PE [19]. However, it may be possible to treat more patients with PE on an outpatient basis because approximately 50% of these patients might have a relatively mild condition such as nonmassive PE with no right ventricular overload [8]. Another report suggested that the readmission rate and prevalence of adverse events were similar in patients admitted for acute PE treatment and those receiving outpatient treatment [22].
Interestingly, our study results suggest that the presence of cancer does not preclude the choice of outpatient treatment; rather, patients with VTE with active cancer have a higher proportion of outpatient treatment. In recent years, the percentage of patients receiving outpatient cancer treatment without hospitalization has been increasing [21]. This may also underlie the fact that patients with VTE, with or without cancer, are treated on an outpatient basis.
Changes in VTE Treatment
Previous studies have suggested that, since the approval of DOACs for the treatment of VTE, warfarin has been replaced as the standard of care not only in Japan [16] but also in many other countries [23,24,25]. A similar trend was confirmed in the present study. There was only a slight difference in the anticoagulant choice depending on the presence or absence of cancer. This suggests that basic treatment strategies do not differ depending on the coexistence of active cancer. In the period covered by our study, the usage rate of unfractionated heparin has remained constant. The drug is used in Japan because low-molecular-weight heparin is not approved for the treatment of VTE. Even in the era when DOACs have become the standard of care, DOACs should not replace heparin in patients with PE with hemodynamic instability or those requiring thrombolytic therapy or thrombectomy. The use of thrombolytic therapy with urokinase or tPA has been decreasing and is currently uncommon. The guidelines [1] do not recommend thrombolytic therapy unless absolutely necessary; the current practice is not far from these recommendations. The same applies to IVC filters. Our results showed that IVC filter placement has been decreasing and has been a rare procedure in the past few years. Guidelines and expert consensus statements [1, 12, 14] recommend IVC filter placement in only very few patients, such as those with contraindications to anticoagulants or at a very high risk of bleeding. Thus, the limited use of IVC filters could be a result of improved adherence to the guidelines.
Dosage of DOACs
In this study, 36.3% of patients with DVT were initiated on anticoagulation with edoxaban alone (Table 2), although edoxaban is supposed to be administered after appropriate initial treatment (e.g., heparin) in the acute phase. However, as mentioned above, patients in the acute phase may have been excluded from the study and may have already passed the acute phase when the thrombus had been found. In such patients and in patients with asymptomatic clots, the need for initial intensified therapy might have been medically determined. Similarly, apixaban and rivaroxaban are supposed to be administered at the standard dose after the initial treatment period with a higher dose, but more than 60% of the patients started at the respective standard dose. This may be because, as with edoxaban, lower doses were chosen for patients whose acute phase had already passed. However, this study could not determine whether patients had acute symptoms or not; therefore, it is not possible to identify which patient characteristics are being treated in this way. Since post-thrombotic syndrome may develop without appropriate initial treatment [26], it is necessary to verify whether these treatment realities improve the prognosis for these patients.
Duration of Anticoagulant Therapy
The guidelines recommend treatment courses of 3 months for patients with “provoked” VTE and ≥ 3 months for patients with “unprovoked” VTE, while long-term treatment (≥ 3 months) is advised for patients with active cancer [1]. It is not clear why the treatment duration observed in the present study was shorter than recommended by the guidelines. One possible reason is that some patients were transferred to other hospitals during the course of their anticoagulation therapy, and subsequent data may not have been captured in the database. Another possibility is that physicians discontinue anticoagulation earlier than suggested by the guidelines in patients at risk of bleeding or in patients with cancer. Data from the COMMAND VTE Registry, a multicenter registry of consecutive acute symptomatic patients with VTE in Japan from 2010 to 2014, also showed that anticoagulation was often discontinued early in patients with active cancer, a finding that differs from current guideline recommendations [27].
In the current study, the duration of treatment with DOACs was longer in patients with PE than in those with DVT and in patients hospitalized for VTE treatment than in those treated for VTE on an outpatient basis. This may indicate that more severely ill patients may be treated for longer periods of time.
Guidelines currently do not clearly define when anticoagulation should be stopped, which may be highly dependent on the judgment of the attending physician. To standardize VTE treatment, further studies are needed to establish the optimal anticoagulation duration based on risk stratification for VTE recurrence and major bleeding. Future studies are expected to confirm whether anticoagulation for a shorter duration than recommended by the guidelines has a negative impact on patient outcomes, and new studies, such as clinical prediction models to determine the risk–benefit balance on the basis of patient background [28], will better define the characteristics of patients who can be safely and effectively treated in an outpatient setting.
Strength and Limitations
The use of the MDV database enabled the analysis of a large number of patients nationwide. Moreover, a retrospective study can help assess actual data that were not influenced by the study objectives. However, similar to most retrospective analyses using claims data, this study has several intrinsic limitations and certain expected biases. First, the data were obtained from hospitals applying the flat-fee payment system, which are mostly large hospitals responsible for acute care. Therefore, the patients analyzed may be in poorer health than the average population and may have more comorbidities. Second, the number of hospitals registered in the MDV database has increased over the years, and this database does not track changes in the number of patients at the same facility over time. However, the temporal trends in the patient population in this study are presented as percentages; thus, this limitation should not significantly affect the interpretation of our study results. Furthermore, the number of registered facilities has been increasing nationwide, and the impact of regional bias is considered to be limited. Third, the claims database does not have detailed clinical and laboratory data on all cases. Disease severity and the size, location, and stability of the thrombi could not be analyzed. Furthermore, the relationship between disease conditions and treatment was not investigated. Fourth, if an outpatient chooses to be seen at a different hospital or an inpatient is transferred to a different hospital, the patient is not considered the same patient, even if those hospitals are listed in the MDV database. Moreover, if a patient is transferred to a medical facility that is not registered in the MDV database, the patient will not be followed up.
Conclusions
In the study period 2011–2018, anticoagulation for VTE was mainly an inpatient treatment, but outpatient treatment has gradually increased, especially after 2015 for both DVT and PE. Half of all VTEs were diagnosed in an outpatient setting, and while an increasing proportion of VTEs was treated in an outpatient setting, the other half were diagnosed during hospitalization for non-VTE treatment. From the viewpoint of medical safety, we suggest that hospital manuals for thrombosis prevention need to be developed and further utilized. It should also be noted that the presence of cancer in patients with VTE does not appear to impede the choice of anticoagulants or outpatient therapy. In general, the duration of anticoagulation was shorter and more variable than that specified by the guidelines. The treatment with DOACs may not always follow the guidelines for initial treatment, such as prior use of heparin or a change to the standard dose after initial high-dose DOAC treatment. In the future, it will be necessary to verify whether these treatment practices lead to improved patient prognosis and prevention of post-thrombotic syndrome. Further research to better define the characteristics of patients who can be treated safely and effectively in an outpatient setting should promote outpatient treatment that contributes to improving patients’ quality of life.
References
Japanese Circulation Society. Guidelines for diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2017). Available from: https://www.j-circ.or.jp/cms/wp-content/uploads/2017/09/JCS2017_ito_h.pdf. Last accessed on 18 June 2022.
Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117(1):19–25. https://doi.org/10.1016/j.amjmed.2004.01.018.
Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. 2007;5(4):692–9. https://doi.org/10.1111/j.1538-7836.2007.02450.x.
Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Predictors of survival after deep vein thrombosis and pulmonary embolism: a population-based, cohort study. Arch Intern Med. 1999;159(5):445–53. https://doi.org/10.1001/archinte.159.5.445.
Prandoni P, Lensing AWA, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1–7. https://doi.org/10.7326/0003-4819-125-1-199607010-00001.
Lee LH, Gallus A, Jindal R, Wang C, Wu CC. Incidence of venous thromboembolism in Asian populations: a systematic review. Thromb Haemost. 2017;117(12):2243–60. https://doi.org/10.1160/TH17-02-0134.
Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98(4):756–64. https://doi.org/10.1160/TH07-03-0212.
Ota S, Matsuda A, Ogihara Y, et al. Incidence, characteristics and management of venous thromboembolism in Japan during 2011. Circ J. 2018;82(2):555–60. https://doi.org/10.1253/circj.CJ-17-0579.
Konstantinides SV, Torbicki A, Agnelli G, et al. Task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–69. https://doi.org/10.1093/eurheartj/ehu283.
Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(Suppl):e419S – e496. https://doi.org/10.1378/chest.11-2301.
Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52. https://doi.org/10.1016/j.chest.2015.11.026.
Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693–738. https://doi.org/10.1182/bloodadvances.2020001830.
Mazzolai L, Aboyans V, Ageno W, et al. Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur Heart J. 2018;39(47):4208–18. https://doi.org/10.1093/eurheartj/ehx003.
Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543–603. https://doi.org/10.1093/eurheartj/ehz405.
Münster AM, Rasmussen TB, Falstie-Jensen AM, et al. A changing landscape: temporal trends in incidence and characteristics of patients hospitalized with venous thromboembolism 2006–2015. Thromb Res. 2019;176:46–53. https://doi.org/10.1016/j.thromres.2019.02.009.
Yamashita Y, Morimoto T, Yoshikawa Y, et al. Temporal trends in the practice pattern for venous thromboembolism in Japan: insight from JROAD-DPC. J Am Heart Assoc. 2020;9(2): e014582. https://doi.org/10.1161/JAHA.119.014582.
Lee CH, Fang CC, Tsai LM, et al. Changing treatment patterns in patients with venous thromboembolism in Taiwan. Circ J. 2020;84(2):283–93. https://doi.org/10.1253/circj.CJ-19-0741.
Schmaier AA, Ambesh P, Campia U. Venous thromboembolism and cancer. Curr Cardiol Rep. 2018;20(10):89. https://doi.org/10.1007/s11886-018-1034-3.
Blom JW, Doggen CJM, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–22. https://doi.org/10.1001/jama.293.6.715.
Yamaguchi Y, Fuji T, Akagi M, et al. The epidemiological study of venous thromboembolism and bleeding events using a Japanese healthcare database—validation study. Jpn J Drug Inform. 2015;17(2):87–93.
The Ministry of Health, Labour and Welfare of Japan. Overview of the 2017 patient survey. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/kanja/17/dl/kanja.pdf. Last accessed on June 19, 2022.
Hendriks SV, Bavalia R, van Bemmel T, et al. Current practice patterns of outpatient management of acute pulmonary embolism: a post-hoc analysis of the YEARS study. Thromb Res. 2020;193:60–5. https://doi.org/10.1016/j.thromres.2020.05.038.
Wändell P, Forslund T, Danin Mankowitz H, et al. Venous thromboembolism 2011–2018 in Stockholm: a demographic study. J Thromb Thrombolysis. 2019;48(4):668–73. https://doi.org/10.1007/s11239-019-01966-y.
Lutsey PL, Walker RF, MacLehose RF, Alonso A, Adam TJ, Zakai NA. Direct oral anticoagulants and warfarin for venous thromboembolism treatment: trends from 2012 to 2017. Res Pract Thromb Haemost. 2019;3(4):668–73. https://doi.org/10.1002/rth2.12222.
Loo SY, Dell’Aniello S, Huiart L, Renoux C. Trends in the prescription of novel oral anticoagulants in UK primary care. Br J Clin Pharmacol. 2017;83(9):2096–106. https://doi.org/10.1111/bcp.13299.
Makedonov I, Kahn SR, Galanaud JP. Prevention and management of the post-thrombotic syndrome. J Clin Med. 2020;9(4):923. https://doi.org/10.3390/jcm9040923.
Sakamoto J, Yamashita Y, Morimoto T, et al. Cancer-associated venous thromboembolism in the real world—from the COMMAND VTE Registry. Circ J. 2019;83(11):2271–81. https://doi.org/10.1253/circj.CJ-19-0515.
Serhal M, Barnes GD. Venous thromboembolism: α clinician update. Vasc Med. 2019;24(2):122–31. https://doi.org/10.1177/1358863X18821159.
Acknowledgements
We would like to thank Editage (http://www.editage.com) for English language editing.
Funding
The funding of this research, including the journal’s Rapid Service was provided by Pfizer Japan Inc. The publication was made available by Bristol Myers Squibb K.K. and Pfizer Japan Inc.
Medical Writing/Editorial Assistance
English language editing was provided by Editage and was funded by Pfizer.
Author Contributions
S. Takahashi and J. Katada designed the paper. S. Takahashi, M. Imura, and J. Katada interpreted the data, critically reviewed the literature, and reviewed and edited the final manuscript.
Disclosures
S. Takahashi, M. Imura, and J. Katada are employees of Pfizer Japan Inc.
Compliance with Ethics Guidelines
This study extracted data that existed in an anonymized structured format and did not contain any personal information of patients. According to applicable legal requirements, such data are not subject to privacy laws. According to the Ethical Guidelines for Human Life Science and Medical Research in Japan, informed consent is not required for studies using unlinkable anonymized data. Therefore, obtaining informed consent from patients and Institutional Review Board approval were not required.
Data Availability
All data generated or analyzed during this study are included in this published article or as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Additional information
Social Media Post: This #observational study examined the #epidemiology and #treatment status of patients with #venous thromboembolism in #Japan.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Takahashi, S., Imura, M. & Katada, J. Epidemiology and Treatment Patterns of Venous Thromboembolism: an Observational Study of Nationwide Time-Series Trends in Japan. Cardiol Ther 11, 589–609 (2022). https://doi.org/10.1007/s40119-022-00284-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-022-00284-4