Abstract
In this paper, the extract of Trigonella seeds was used as sensitizer for dye-sensitized solar cells (DSSCs). The natural dye was extracted from the seeds using water and alcohol as solvents for the raw material. The UV–Vis absorption spectra of Trigonella extract solution and dye adsorbed on TiO2 film were measured. DSSCs sensitized by Trigonella extracted using water as a solvent exhibited better performance with efficiency of 0.215 %. The performance of the fabricated DSSCs was attempted to enhance by acid treatment of the FTO substrates with HNO3, H3PO4, and H2SO4. Electrochemical impedance spectroscopy of the fabricated cells was also carried out.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
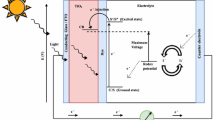
Nowadays, the world is looking forward for obtaining clean renewable alternative energy source which is essential for developing our current lifestyle and economic growth. This was an inevitable result of the imbalance between the production and consumption of fossil fuel. Solar energy was one of the most promising technologies that have been extensively studied. The outcome of these studies was construction of three generations of solar cells. The bulk crystalline-silicon solar cell was the first generation which has an acceptable efficiency, but its high cost and industrial requirements led to the second generation known as thin film solar cells. The second generation is cheaper but has low efficiency, so the third generation came to keep the benefits of easy manufacturing and low cost while looking for better converging efficiency. Dye-sensitized solar cells (DSSCs) invented by O’Regan and Grätzel [1] are famous by their simple preparation process accompanied with a reasonable efficiency. The main structure of this kind of solar cells is built of a photoactive electrode consisting of a tin-oxide conducting glass mounted by a nanostructured wide band gap metal-oxide semiconductor thin film anchored to suitable dye molecules, which have the ability to be excited by absorbing light photons. The excited electrons are injected from the dye lowest unoccupied molecular orbital (LUMO) to the semiconductor conduction band, passing through an external load to a platinum counter electrode. To complete the circuit the photoactive and the counter electrodes are sandwiched with the space between them is filled by an iodide–triiodide electrolyte. The main rule of the electrolyte is to regenerate the oxidized dye. Conversely, the electrolyte will be regenerated from the counter electrode. Each component of this simple structure has been extensively discussed by many researchers seeking for efficiency improvement. Many wide band gap semiconductors like (TiO2, ZnO, SnO2) were studied with different morphologies (nanopowder, nanotubes, nanowires) [2–4]. Furthermore, the vital rule for the dye in the working mechanism of DSSCs in light harvesting was investigated. Many chemical groups like homoleptic dyes (N3, N719, N749) developed by Grätzel [5, 6] heteroleptic sensitizers (Z907, K19, K77), ruthenium polypyridyl complexes, and organic dyes (triphenylamine, carbazole, phenothiazine) have recorded encouraging efficiencies up to 10–12 %. The high cost and environmental harms of these dyes turned the attention toward natural pigments like anthocyanins, chlorophylls, and hypericin [7–9] extracted from plant leaves, roots, flowers, seeds, and fruits [10–16]. In spite of natural dyes low efficiencies compared to ruthenium complexes, they attracted the attention of many researchers working on enhancing their performance by studying the effect of various factors in the extraction process, such as drying, grinding, temperature, anchorage time, and using different compounds of solvents [17, 18]. Natural juglone dye was obtained from the outer shell of walnut fruit by hot extraction method followed by doping using aluminium, copper and iron metals and used as a photosensitizer of DSSCs [19]. Fast production of the ZnO nanorods by bottom up approach using arc discharge method in de-ionized water was carried out for DSSC applications [20]. The synthesis of copper-doped polyaniline (NPANI-Cu-X) was performed in many solvents [21]. The obtained PANI was employed as counter electrode in a DSSC configuration. DSSC bilayer design was developed using an Fe2+/Fe3+ (ferrocene) liquid electrolyte and natural dyes extracted from Hypericum perforatum, Rubia tinctorum L. and Reseda luteola [22].
Dye-sensitized solar cells have been prepared using natural dyes such as red cabbage, red perilla, rosella, blue pea, and curcumin [23]. The efficiency of the fabricated DSSCs was improved by selecting a proper sensitizer and modifying the surface of FTO by chemical treatment. The FTO surface was treated by HCl and TiCl4. Moreover, different organic solvents were used to enhance the extent of sensitization [23].
In this work, the natural dye extracted from Trigonella seeds was studied as a photosensitizer of DSSCs. The absorption spectrum of the extract was determined. The effect of using alcohol and water as solvents of the Trigonella seeds in the dye extraction was investigated. Pre-acidic treatment to the conductive glass substrates was conducted to improve the DSSC photovoltaic performance. Impedance spectroscopy study was provided to understand the transport of electron and hole carriers, interfacial charge transfer and recombination.
Experimental
Dye preparation
The clean dry seeds of Trigonella plant (1st washed with distilled water and dried at 70 °C) were grinded by a mortar to form a fine powder. 1 g of the fine powder was immersed in 5 ml ethyl alcohol at room temperature and left in dark for 24 h, and then the solution was filtered to obtain the final extract solution. The same procedure was repeated for the same amount of Trigonella fine powder but the solvent was replaced with distilled water. Finally, the obtained extracts were kept in dark cold place.
Photoelectrode preparation
A transparent conducting FTO sheets (resistance 12–14 Ω/cm2; transmittance: 82–84 %; Xinyan Technology Ltd, Hong Kong) with dimensions 1 cm × 1.5 cm were cleaned using ultrasonic bath filled with detergent solution for 20 min, then rinsed with distilled water and dried.
The FTO conductive glass sheets were divided into four groups. The first group was not treated by acids. The second, third, and fourth groups were immersed for 10 min in acid solutions of HNO3, H2SO4 and H3PO4, respectively, each of 0.1 M concentration.
0.2 grams of TiO2 nanopowder (P25) was added to 0.1 g polyethylene glycol and the mixture was grinded for 30 min until a fine paste is obtained.
The TiO2 paste was spread on all the FTO groups by doctor-blade technique, where a thin film was obtained on an area of 0.25 cm2. The sintering process started by drying the sheets at 70 °C for 20 min, raising temperature to 180 °C for 10 min, then 450 °C for 40 min. After cooling down to 70 °C, the TiO2 thin film samples were soaked in the Trigonella natural extract for 24 h in the dark.
Assembling of DSSC
The prepared TiO2 photoelectrode (anode) and a conductive glass sheet plated with platinum by electrodeposition (cathode) were sandwiched to assemble the DSSC. Finally, the space between the two electrodes was filled with a liquid electrolyte solution (I −/I −3 ) composed of 2 ml acetonitrile (ACN), 8 ml propylene carbonate (p-carbonate), 0.668 g potassium iodide, KI, and 0.0634 g iodine, I2. Then, the two electrodes were clipped together to form a solar cell ready for light exposure.
Measurement
The UV–Vis absorption spectra of Trigonella dye extracted by water and alcohol were measured using Thermoline Genesys 6 spectrophotometer with wavelength range extending from 350 to 800 nm. The representative UV–Vis absorption spectrum of the anchored Trigonella extract on TiO2 thin film layer was measured by collecting a diffuse reflectance spectrum with a V-670, JASCO spectrophotometer then transforming it into absorption spectrum according to the Kubelka–Munk relationship. Moreover, the cyclic voltammetry was used for the measurement of the HOMO, LUMO, and energy band gap. The current density–voltage (J–V) characteristic curves of the DSSCs under study were measured using National Instruments data acquisition card (USB NI 6251) in combination of a Labview program. An applied voltage in the range between −1 and 1 V was applied to the illuminated solar cell during current–voltage measurements. The J–V curves were measured at 100 mW/cm2 irradiations using high pressure mercury arc lamp. Electrochemical impedance spectroscopy (EIS) was carried out using an SP-200 potentiostat (Biologic, USA), with the frequency ranging from 100 Hz to 200 kHz.
Results and discussion
Absorption spectra
Trigonella extract UV–Vis absorption spectra in both water and alcohol as solvents are shown in Fig. 1. According to the figure, a clear absorption peak was observed at 400 nm in case of alcohol and another peak at 444 nm for water. A diffuse reflectance spectrum was collected for the Trigonella dye adsorbed on TiO2 film and transformed to the absorption spectrum according to the Kubelka–Munk relationship [24], F(R) = (1 – R)2/2R = k/s = Ac/s where R is the reflectance, k is the absorption coefficient, s is the scattering coefficient, A is the absorbance and c is the concentration of absorbing species. When c and s remain the same, the spectrum obtained for Kubelka–Munk function can be considered as absorbance. Figure 2 shows the detected absorption peak at 430 nm for the dye adsorbed on TiO2 which indicates a red shift towards higher wavelength compared to that of the extract in solution.
The cyclic voltammetry was used for the measurement of the HOMO, LUMO, and energy band gap. It was found that E HOMO = 6.28 eV, E LUMO = 3.75 eV and E opt = 2.5 eV.
Photovoltaic properties
The J–V characteristic curves for DSSCs (fabricated using untreated FTO, 1st group,) sensitized by the natural extract of Trigonella seeds using water and alcohol as solvents are illustrated in Fig. 3. The main photoelectrochemical parameters obtained from Fig. 3 are listed in Table 1. These parameters are short circuit current J sc, open circuit voltage V oc, fill-factor FF, and conversion efficiency η. It is clear from the figure that the performance of DSSCs sensitized by Trigonella extracted using water as a solvent is much better than that of solar cells sensitized by Trigonella extracted with alcohol. Referring to Table 1, J sc = 0.371 mA/cm2 with alcohol and J sc = 0.747 mA/cm2 with water giving 201 % improvement. On the other hand, V oc = 0.599 V and FF = 48 % with alcohol are almost the same as V oc = 0.601 V and FF = 48 % with water, which means that the raise in efficiency from η = 0.106 % with alcohol up to η = 0.215 % with water leading to 202.8 % improvement is due to the significant improvement in the short circuit current. It is worth mentioning that the extracts of water are different from those of alcohol. The current improvement can be attributed to the extracts of water.
Acidic treatment of FTO sheets
The DSSCs used in this treatment were sensitized with the natural extract of Trigonella seeds using water as a solvent. The J–V characteristic curves of DSSCs fabricated using untreated FTO (1st group) and those prepared using acid treated FTO (2nd, 3rd, and 4th groups) are plotted in Fig. 4. Their photoelectrochemical parameters are listed in Table 2. According to the J–V curves we can notice that the FTO treatment with HNO3 and H3PO4 acids gave better performance than untreated cells. From Table 2, the treatment with HNO3 gave a short circuit current J sc = 0.840 mA/cm2, V oc = 0.641 V, and η = 0.259 %, leading to an improvement by 112.5, 106.6, and 120.4 %, respectively, over the untreated cells. On the other hand, the enhancements in case of H3PO4 were only 101.2 % for J sc and 100.9 % for V oc. This improvement is due to better adhesion of the TiO2 layer to the FTO, which means reducing the resistivity for high charge transport leading to higher short-circuit current density.
Impedance spectroscopy measurements
The solar cells sensitized with the extract of Trigonella seeds were perturbed by a small AC voltage signal of amplitude 10 mV with varying frequency (100 Hz to 200 kHz), to carry out an EIS study. This EIS study was employed to figure out the charge transport kinetics through the analysis of the Nyquist and Bode plots shown in Figs. 5 and 6, respectively. The main parameters calculated from the curves are the charge recombination resistance R S, the charge transfer resistance R CT, double layer capacitance (C dl), the constant phase element CPE (α) due to double layer capacitance, and the effective lifetime of electrons τ. All these parameters were calculated and summarized in Table 3. As can be seen from the table, R S = 31 Ω which is small enough for fast electron transport, in the same context a very large R CT = 7,204 Ω indicates long lifetime (62.5 ms) for the electrons in the film. The CPE coefficient α = 0.76 illustrates electrode surface roughness accompanied with complicated structure double layer capacitance.
Conclusions
The extract of Trigonella seeds as a natural sensitizer was used in the preparation of DSSCs. Two different solvents (alcohol and water) were used in the extraction process of the dye from the raw material. The highest conversion efficiency of 0.215 % (with water as a solvent) compared to 0.106 % (with alcohol as a solvent) was obtained for the fabricated DSSC. A pre-treatment process for the FTO glass substrates with acidic solutions led to an efficiency improvement in case of HNO3 and H3PO4. Impedance spectroscopy study for the fabricated solar cells was obtained from the analysis of the Nyquist and Bode plots, and the most important parameters like charge recombination resistance R S, charge transfer resistance R CT, constant phase element CPE due to double layer capacitance (C dl), and effective lifetime of electrons τ were determined.
References
O’Regan, B., Grätzel, M.: A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353, 737–740 (1991)
Roy, M., Balraju, P., Kumar, M., Sharma, G.: Dye-sensitized solar cell based on rose bengal dye and nanocrystalline TiO2. Sol. Energy Mater. Sol. Cells 92, 909–913 (2008)
Gubbala, S., Chakrapani, V., Kumar, V., Sunkara, M.K.: Band-edge engineered hybrid structures for dye-sensitized solar cells based on SnO2 nanowires. Adv. Funct. Mater. 18, 2411–2418 (2008)
El-Agez, T., El Tayyan, A., Al-Kahlout, A., Taya, S., Abdel-Latif, M.: Dye-sensitized solar cells based on ZnO films and natural dyes. Int. J. Mater. Chem. 2, 105–110 (2012)
Grätzel, M.: Dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 4, 145–153 (2003)
Grätzel, S.: Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 44, 20–28 (2005)
Dai, O., Rabani, I.: Photosensitization of nanocrystalline TiO2 films by anthocyanin dyes. J. Photochem. Photobiol. A Chem. 26, 421–429 (2002)
Changa, H., Wu, H., Chen, T., Huang, K., Jwo, C., Lo, Y.: Dye-sensitized solar cell using natural dyes extracted from spinach and ipomoea. J. Alloys Compd. 495, 606–610 (2010)
Wongcharee, K., Meeyoo, V., Chavadej, S.: Dye-sensitized solar cell using natural dyes extracted from rosella and blue pea flowers. Sol. Energy Mater. Sol. Cells 91, 566–571 (2007)
Abdel-Latif, M., Abuiriban, M., El-Agez, T., Taya, S.: Dye-sensitized solar cells using dyes extracted from flowers, leaves, parks, and roots of three trees. Int. J. Renew. Energy Res. 5, 294–298 (2015)
El-Ghamri, H., El-Agez, T., Taya, S., Abdel-Latif, M., Batniji, A.: Dye-sensitized solar cells with natural dyes extracted from plant seeds. Mater. Sci. Pol. 32, 547–554 (2015)
Taya, S., El-Agez, T., El-Refi, K., Abdel-Latif, M.: Dye-sensitized solar cells based on dyes extracted from dried plant leaves. Turk. J. Phys. 39, 24–30 (2015)
Calogero, G., Marco, G.: Red Sicilian orange and purple eggplant fruits as natural sensitizers for dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 92, 1341–1346 (2008)
Batniji, A., Morjan, R., Abdel-Latif, M., El-Agez, T., Taya, S., El-Ghamri, H.: Aldimine derivatives as photosensitizers for dye-sensitized solar cells. Turk. J. Phys. 38, 86–90 (2014)
Taya, S., El-Agez, T., Abdel-Latif, M., El-Ghamri, H., Batniji, A., El-Sheikh, I.: Fabrication of dye-sensitized solar cells using dried plant leaves. Int. J. Renew. Energy Res. 4, 384–388 (2014)
El-Agez, T., Taya, S., ElRefi, K., Abdel-Latif, M.: Dye sensitized solar cells using some organic dyes as photosensitizers. Optica Applicata 44, 345–351 (2014)
Hemmatzadeh, H., Mohammadi, A.: Improving optical absorptivity of natural dyes for fabrication of efficient dye-sensitized solar cells. J. Theor. Appl. Phys. 7, 57–65 (2013)
Dumbravă, A., Enache, I., Oprea, C., Georgescu, A., Gîrţu, M.: Toward a more efficient utilization of betalains as pigments for dye-sensitized solar cell. Dig. J. Nanomater. Biostruct. 7, 339–351 (2012)
Sönmezoğlu, S., Akyürek, C., Akış, H.: Modification of juglone dye as a sensitiser in dye-sensitised solar cells. IET Optoelectron. 8, 270–276 (2014)
Sönmezoğlua, S., Eskizeybekb, V., Toumiatc, A., Avcıd, A.: Fast production of ZnO nanorods by arc discharge in de-ionized water and applications in dye-sensitized solar cells. J. Alloys Compd. 586, 593–599 (2014)
Recep, T., Mahir, G., Muzaffer, C., Sönmezoğlu, S.: Effects of solvent and copper-doping on polyaniline conducting polymer and its application as a counter electrode for efficient and cost-effective dye-sensitized solar cells. Synth. Met. 212, 75–83 (2015)
Sönmezoğlu, S., Akyurek, C., Akin, S.: High-efficiency dye-sensitized solar cells using ferrocene-based electrolytes and natural photosensitizers. J. Phys. D Appl. Phys. 45, 425101 (2012)
Sreekala, C., Jinchu, I., Sreelatha, K., Janu, Y., Prasad, N., Kumar, M., Sadh, A., Roy, M.: Influence of solvents and surface treatment on photovoltaic response of DSSC based on natural curcumin dye. IEEE J. Photovolt. 2, 312–319 (2012)
Shen, J., Li, Y., He, J.: On the Kubelka–Munk absorption coefficient. Dyes Pigments 127, 187–188 (2016)
Acknowledgments
The authors would like to express gratitude to the ministry of higher education for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Batniji, A., Abdel-Latif, M.S., El-Agez, T.M. et al. Dyes extracted from Trigonella seeds as photosensitizers for dye-sensitized solar cells. J Theor Appl Phys 10, 265–270 (2016). https://doi.org/10.1007/s40094-016-0225-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40094-016-0225-9