Abstract
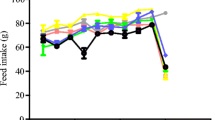
Terminalia arjuna is well known for its health benefits. It is used as a traditional medicine to cure many disease conditions. In the present study, the authors have evaluated the cardioprotective potential of T. arjuna extract (TAE) against isoproterenol (ISO)-induced cardiac stress in male Wistar albino rats. TAE 50 and 75 mg/kg body weight significantly (p < 0.01) protected rats against ISO (85 mg/kg body weight)-induced stress and were evidenced by electrocardiography and serum biomarker enzyme levels. Pretreatment with TAE-protected oxidative stress-induced cardiac stress by decreasing the generation of free radical like ROS, hydroperoxide and nitrite levels. TAE pretreatment retained cardiac rhythm, myocardial membrane integrity and reduced the rate of lipid peroxidation induced by ISO treatment. TAE pretreatment also elevated antioxidant enzymes (superoxide dismutase, catalase, glutathione reductase and glutathione peroxidase) and total glutathione levels against ISO-induced cardiac stress. Further, anti-apoptotic effects of TAE were confirmed by immunoblotting studies by alleviating the phosphorylation of JNK, c-jun and also by regulating protein expressions of Bax, Bcl2 and iNOS levels in myocardium. Overall results suggest that the TAE extract exhibits antioxidant and anti-apoptotic defense mechanisms against ISO-induced cardiac stress in Wistar albino rats.




Similar content being viewed by others
References
Sugamura K, Keaney JF Jr (2011) Reactive oxygen species in cardiovascular disease. Free Radic Biol Med 51:978–992
He F, Zuo L (2015) Redox roles of reactive oxygen species in cardiovascular diseases. Int J Mol Sci 16:27770–27780
Yong J, Lin D, Tan XR (2017) Primary prevention of cardiovascular disease in older adults in China. World J Clin Cases 5:349–359
Biswas M, Biswas K, Karan TK, Bhattacharya S, Ghosh AK, Haldar PK (2011) Evaluation of analgesic and anti-inflammatory activities of Terminalia arjuna leaf. J Phytol 3:33–38
Maulik SK, Talwar KK (2012) Therapeutic potential of Terminalia arjuna in cardiovascular disorders. Am J Cardiovasc Drugs 12(3):157–163
Khaliq F, Fahim M (2018) Role of Terminalia Arjuna in improving cardiovascular functions: a review. Indian J Physiol Pharmacol 62(1):8–19
Ghosh J, Sil PC (2013) Arjunolic acid: a new multifunctional therapeutic promise of alternative medicine. Biochimie 95:1098–1109
Parveen A, Babbar R, Agarwal S, Kotwani A, Fahim M (2011) Mechanistic clues in the cardioprotective effect of Terminalia arjuna bark extract in isoproterenol-induced chronic heart failure in rats. Cardiovasc Toxicol 11:48–57
Khan ZH, Faruquee HM, Shaik MM (2013) Phytochemistry and pharmacological potential of Terminalia arjuna L. Med Plant Res 3:70–78
Chander R, Singh K, Khanna AK, Kaul SM, Puri A, Saxena R, Bhatia G, Rizvi F, Rastogi AK (2004) Antidyslipidemic and antioxidant activities of different fractions of Terminalia arjuna stem bark. Indian J Clin Biochem 19:141
Bansal T, Chatterjee E, Singh J, Ray A, Kundu B, Thankamani V, Sengupta S, Sarkar S (2017) Arjunolic acid, a peroxisome proliferator-activated receptor α agonist, regresses cardiac fibrosis by inhibiting non-canonical TGF-β signaling. J Biol Chem 292:16440–16462
Siddiqui MA, Ahmad U, Khan AA, Ahmad M, Badruddeen, Khalid M, Akhtar J (2016) Isoprenaline: a tool for inducing myocardial infarction in experimental animals. Int J Pharm 6(2):138–144
Upaganlawar A, Gandhi H, Balaraman R (2011) Isoproterenol induced myocardial infarction: protective role of natural products. J Pharmacol Toxicol Methods 6:1–17
Ashok kumar R, Jamuna S, Sadullah MS, Devaraj SN (2018) Vitexin protects isoproterenol induced post myocardial injury by modulating hipposignaling and ER stress responses. Biochem Biophys Res Commun 496:731–737
Zhang HJ, Chen RC, Sun GB, Yang LP, Xu XD, Sun XB (2018) Protective effects of total flavonoids from Clinopodium chinense (Benth.) O. Ktze on myocardial injury in vivo and in vitro via regulation of Akt/Nrf2/HO-1 pathway. Phytomedicine 40:88–97
Yazdanyar A, Newman AB (2009) The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin Geriatr Med 25:563–577
Peliciari-Garcia RA, Darley-Usmar V, Young ME (2018) An overview of the emerging interface between cardiac metabolism, redox biology and the circadian clock. Free Radic Biol Med 119:75–84
Csanyi G, Miller F J Jr (2014) Oxidative stress in cardiovascular disease. Int J Mol Sci 15:6002–6008
Ramond A, Godin-Ribuot D, Ribuot C, Totoson P, Koritchneva I, Cachot S, Levy P, Joyeux-Faure M (2013) Oxidative stress mediates cardiac infarction aggravation induced by intermittent hypoxia. Fundam Clin Pharmacol 27:252–261
Takimoto E, Kass DA (2007) Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49:241–248
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Socci DJ, Bjugstad KB, Jones HC, Pattisapu JV, Arendash GW (1999) Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp Neurol 155(1):109–117
Deiana M, Aruoma OI, Maria de Lourdes PB, Spencer JPE, Kaur H, Halliwell B, Aeschbach R, Banni S, Assunta Dessi M, Corongiu FP (1999) Inhibition of peroxynitrite dependent DNA base modification and tyrosine nitration by the extra virgin olive oil-derived antioxidant hydroxytyrosol. Free Radic Biol Med 26(5–6):762–769
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. In: Abelson JN (ed) Methods in enzymology, vol 52. Academic Press, London, pp 302–310
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. In: Glazer HSA (ed) Methods in enzymology, vol 233. Academic Press, London, pp 357–363
Aebi H (1984) Catalase in vitro. In: Kaplan NP (ed) Methods in enzymology, vol 105. Academic Press, London, pp 121–126
Aykac G, Uysal M, Yalçin AS, Kocak-Toker N, Sivas A, Oz H (1985) The effect of chronic ethanol ingestion on hepatic lipid peroxide, glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicology 36:71–76
Judd JT, Wexler BC, Williamson G, Bickers M, Springs M (1969) Myocardial connective tissue metabolism in response to injury: histological and chemical studies of mucopolysaccharide and collagen in rat hearts after isoproterenol-induced infarction. Circ Res 25:201–214
Rajadurai M, Prince PSM (2006) Preventive effect of naringin on lipid peroxides and antioxidants in isoproterenol-induced cardiotoxicity in Wistar rats: biochemical and histopathological evidences. Toxicology 228:259–268
Li X, Wang X, Guo Y, Deng N, Zheng P, Xu Q, Wu Y, Dai G (2012) Regulation of endothelial nitric oxide synthase and asymmetric dimethylarginine by matrine attenuates isoproterenol-induced acute myocardial injury in rats. J Pharm Pharmacol 64:1107–1118
Derbali A, Mnafgui K, Affes M, Derbali F, Hajji R, Gharsallah N, Allouche N, El Feki A (2015) Cardioprotective effect of linseed oil against isoproterenol-induced myocardial infarction in Wistar rats: a biochemical and electrocardiographic study. J Physiol Biochem 71:281–288
Li H, Xie YH, Yang Q, Wang SW, Zhang BL, Wang JB, Cao W, Bi LL, Sun JY, Miao S, Hu J (2012) Cardioprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats. PLoS ONE 7:1–10
Mazzoleni A, Curtin ME, Wolff R, Reiner L, Somes G (1975) On the relationship between heart weights, fibrosis, and QRS duration. J Electrocardiol 8:233–236
Akdeniz B, Guneri S, Savas IZ, Aslan O, Baris N, Badak O, Kirimli O, Goldeli O (2006) Effects of carvedilol therapy on arrhythmia markers in patients with congestive heart failure. Int Heart J 47:565–573
Patel JS, Setty SK, Chakraborty M, Kamath JV (2012) Evaluation of cardioprotective activity of Medohar vati by isoproterenol induced myocardial damage in rats. Int Res J Pharm 3:214–217
Zaafan MA, Zaki HF, El-Brairy AI, Kenawy SA (2013) Protective effects of atorvastatin and quercetin on isoprenaline-induced myocardial infarction in rats. Bull Fac Pharm 51:35–41
Khaliq F, Parveen A, Singh S, Hussain ME, Fahim M (2013) Terminalia arjuna improves cardiovascular autonomic neuropathy in streptozotocin-induced diabetic rats. Cardiovasc Toxicol 13:68–76
Manu TM, Anand T, Pandareesh MD, Kumar PB, Khanum F (2019) Terminalia arjuna extract and arjunic acid mitigate cobalt chloride–induced hypoxia stress–mediated apoptosis in H9c2 cells. Naunyn-Schmiedeberg’s Arch Pharmacol 392:1107–1119
Rodrigo R, Libuy M, Feliú F, Hasson D (2013) Oxidative stress-related biomarkers in essential hypertension and ischemia-reperfusion myocardial damage. Dis Markers 35:773–790
Sivalokanathan S, Ilayaraja M, Balasubramanian MP (2006) Antioxidant activity of Terminalia arjuna bark extract on N-nitrosodiethylamine induced hepatocellular carcinoma in rats. Mol Cell Biochem 281:87–95
Ly JD, Grubb DR, Lawen A (2003) The mitochondrial membrane potential (Δψ m) in apoptosis; an update. Apoptosis 8:115–128
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329(1–2):23–38
Cai J, Yang J, Jones D (1998) Mitochondrial control of apoptosis: the role of biochimica et biophysica acta. Bioenergetics 1366:139–149
Ishibashi Y, Takahashi N, Tokumaru A, Karino K, Sugamori T, Sakane T, Kunizawa Y, Yoshitomi H, Sato H, Oyake N, Murakami Y (2008) Activation of inducible NOS in peripheral vessels and outcomes in heart failure patients. J Cardiac Fail 14:724–731
Buttros JB, Bergamaschi CT, Ribeiro DA, Fracalossi AC, Campos RR (2009) Cardioprotective actions of ascorbic acid during isoproterenol-induced acute myocardial infarction in rats. Pharmacology 84:29–37
Zhang W, Mottillo EP, Zhao J, Gartung A, VanHecke GC, Lee JF, Maddipati KR, Xu H, Ahn YH, Proia RL, Granneman JG (2014) Adipocyte lipolysis-stimulated interleukin-6 production requires sphingosine kinase 1 activity. J Biol Chem 289:32178–32185
Zhang Y, Xu J, Long Z, Wang C, Wang L, Sun P, Li P, Wang T (2016) Hydrogen (H2) inhibits isoproterenol-induced cardiac hypertrophy via antioxidative pathways. Front Pharmacol 7:1–12
Kumar S, Enjamoori R, Jaiswal A, Ray R, Seth S, Maulik SK (2009) Catecholamine-induced myocardial fibrosis and oxidative stress is attenuated by Terminalia arjuna (Roxb.). J Pharm Pharmacol 61:1529–1536
Wang RS, Oldham WM, Loscalzo J (2014) Network-based association of hypoxia-responsive genes with cardiovascular diseases. New J Phys 16:1–22
Acknowledgements
The authors are grateful to Director DFRL for providing constant support and necessary facilities to conduct the research work. Mohan Manu T is thankful to DST for providing INSPIRE Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance statement Cardiovascular diseases (CVDs) are considered as the major cause of mortality throughout the world, impairment of heart and circulatory system results in CVDs. Plant-based components have a long-term protective effect and economical.
Rights and permissions
About this article
Cite this article
Thangaraju, M., Tamatam, A., Bhat, P.V. et al. Terminalia arjuna Extract Attenuates Isoproterenol-Induced Cardiac Stress in Wistar Rats via an Anti-Apoptotic Pathway. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 90, 1101–1112 (2020). https://doi.org/10.1007/s40011-020-01180-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-020-01180-4