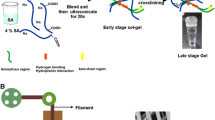
Abstract
Background:
Bioglasses are used in applications related to bone rehabilitation and repair. The mechanical and bioactive properties of polysaccharides like alginate and agarose can be modulated or improved using bioglass nanoparticles. Further essential metal ions used as crosslinker have the potential to supplement cultured cells for better growth and proliferation.
Method:
In this study, the alginate bioink is modulated for fabrication of tissue engineering scaffolds by extrusion-based 3D bioprinting using agarose, bioglass nanoparticles and combination of essential trace elements such as iron, zinc, and copper. Homogeneous bioink was obtained by in situ mixing and bioprinting of its components with twin screw extruder (TSE) based 3D bioprinting, and then distribution of metal ions was induced through post-printing diffusion of metal ions in the printed scaffolds. The mechanical and 3d bioprinting properties, microscopic structure, biocompatibility of the crosslinked alginate/agarose hydrogels were analyzed for different concentrations of bioglass. The adipose derived mesenchymal stem cells (ADMSC) and osteoblast cells (MC3T3) were used to evaluate this hydrogel’s biological performances.
Results:
The porosity of hydrogels significantly improves with the incorporation of the bioglass. More bioglass concentration results in improved mechanical (compressive, dynamic, and cyclic) and 3D bioprinting properties. Cell growth and extracellular matrix are also enhanced with bioglass concentration.
Conclusion:
For bioprinting of the bioinks, the advanced TSE head was attached to 3D bioprinter and in situ fabrication of cell encapsulated scaffold was obtained with optimized composition considering minimal effects on cell damage. Fabricated bioinks demonstrate a biocompatible and noncytotoxic scaffold for culturing MC3T3 and ADMSC, while bioglass controls the cellular behaviors such as cell growth and extracellular matrix formation.
Graphical abstract








Similar content being viewed by others
Data availability
The data presented in this study are available on request from all the authors.
References
Janarthanan G, Noh I. Recent trends in metal ion based hydrogel biomaterials for tissue engineering and other biomedical applications. J Mater Sci Technol. 2021;63:35–53.
Gopinathan J, Noh I. Click chemistry-based injectable hydrogels and bioprinting inks for tissue engineering applications. Tissue Eng Regen Med. 2018;15:531–46.
Yang Y, Xu L, Wang J, Meng Q, Zhong S, Gao Y, et al. Recent advances in polysaccharide-based self-healing hydrogels for biomedical applications. Carbohydr Polym. 2022;283:119161.
Nie J, Wang Z, Hu Q. Chitosan Hydrogel structure modulated by metal ions. Sci Rep. 2016;6:36005.
Raia NR, Partlow BP, McGill M, Kimmerling EP, Ghezzi CE, Kaplan DL. Enzymatically crosslinked silk-hyaluronic acid hydrogels. Biomaterials. 2017;131:58–67.
Khanmohammadi M, Jalessi M, Asghari A. Biomimetic hydrogel scaffolds via enzymatic reaction for cartilage tissue engineering. BMC Res Notes. 2022;15:174.
López-Marcial GR, Zeng AY, Osuna C, Dennis J, García JM, O'Connell GD. Agarose-Based Hydrogels as Suitable Bioprinting Materials for Tissue Engineering. ACS Biomater Sci Eng. 2018;4:3610–6.
Hatton J, Davis GR, Mourad AI, Cherupurakal N, Hill RG, Mohsin S. Fabrication of porous bone scaffolds using alginate and bioactive glass. J Funct Biomater. 2019;10:15.
Wu C, Zhang Z, Zhou K, Chen W, Tao J, Li C, et al. Preparation and characterization of borosilicate-bioglass-incorporated sodium alginate composite wound dressing for accelerated full-thickness skin wound healing. Biomed Mater. 2020;15:055009.
Bargavi P, Ramya R, Chitra S, Vijayakumari S, Riju Chandran R, Durgalakshmi D, et al. Bioactive, degradable and multi-functional three-dimensional membranous scaffolds of bioglass and alginate composites for tissue regenerative applications. Biomater Sci. 2020;8:4003–25.
Zamani D, Moztarzadeh F, Bizari D. Alginate-bioactive glass containing zn and mg composite scaffolds for bone tissue engineering. Int J Biol Macromol. 2019;137:1256–67.
Thomas A, Johnson E, Agrawal AK, Bera J. Preparation and characterization of glass–ceramic reinforced alginate scaffolds for bone tissue engineering. J Mater Res. 2019;34:3798–809.
El-Kady AM, Ali AA, El-Fiqi A. Controlled delivery of therapeutic ions and antibiotic drug of novel alginate-agarose matrix incorporating selenium-modified borosilicate glass designed for chronic wound healing. J Non Cryst Solids. 2020;534:119889.
Gao L, Zhou Y, Peng J, Xu C, Xu Q, Xing M, et al. A novel dual-adhesive and bioactive hydrogel activated by bioglass for wound healing. NPG Asia Mater. 2019;11:16.
Qi Q, Zhu Y, Liu G, Yuan Z, Li H, Zhao Q. Local intramyocardial delivery of bioglass with alginate hydrogels for post-infarct myocardial regeneration. Biomed Pharmacother. 2020;129:110382.
Massana Roquero D, Othman A, Melman A, Katz E. Iron(iii)-cross-linked alginate hydrogels: a critical review. Mater Adv. 2022;3:1849–73.
Zeng Q, Han Y, Li H, Chang J. Design of a thermosensitive bioglass/agarose-alginate composite hydrogel for chronic wound healing. J Mater Chem B. 2015;3:8856–64.
Falah SNM, Al-Fartusie S. Essential trace elements and their vital roles in human body. Indian J Adv Chem Sci. 2017;5:127–36.
Dravid A, McCaughey-Chapman A, Raos B, O’Carroll SJ, Connor B, Svirskis D. Development of agarose-gelatin bioinks for extrusion-based bioprinting and cell encapsulation. Biomed Mater. 2022. https://doi.org/10.1088/1748-605X/ac759f.
Oliver-Ferrándiz M, Milián L, Sancho-Tello M, Martín de Llano JJ, Gisbert Roca F, Martínez-Ramos C, et al. Alginate-agarose hydrogels improve the in vitro differentiation of human dental pulp stem cells in chondrocytes. A histological study. Biomedicines. 2021;9:834.
Cañaveral S, Morales D, Vargas AF. Synthesis and characterization of a 58S bioglass modified with manganese by a sol-gel route. Mater Lett. 2019;255:126575.
da Fonseca GF, Avelino SOM, Mello DCR, do Prado RF, Campos TMB, de Vasconcellos LMR, et al. Scaffolds of PCL combined to bioglass: synthesis, characterization and biological performance. J Mater Sci Mater Med. 2020;31:41.
Peter M, Binulal NS, Nair SV, Selvamurugan N, Tamura H, Jayakumar R. Novel biodegradable chitosan–gelatin/nano-bioactive glass ceramic composite scaffolds for alveolar bone tissue engineering. Chem Eng J. 2010;158:353–61.
Xia D, Wang Y, Wu R, Zheng Q, Zhang G, Xu S, et al. The effect of pore size on cell behavior in mesoporous bioglass scaffolds for bone regeneration. Appl Mater Today. 2022;29:101607.
Zeng Q, Han Y, Li H, Chang J. Bioglass/alginate composite hydrogel beads as cell carriers for bone regeneration. J Biomed Mater Res B Appl Biomater. 2014;102:42–51.
Wers E, Lefeuvre B. New hybrid agarose/Cu-Bioglass® biomaterials for antibacterial coatings. Korean J Chem Eng. 2017;34:2241–7.
Bhattacharyya A, Ham HW, Sonh J, Gunbayar M, Jeffy R, Nagarajan R, et al. 3D bioprinting of complex tissue scaffolds with in situ homogeneously mixed alginate-chitosan-kaolin bioink using advanced portable biopen. Carbohydr Polym. 2023;317:121046.
Bhattacharyya A, Janarthanan G, Noh I. Nano-biomaterials for designing functional bioinks towards complex tissue and organ regeneration in 3D bioprinting. Addit Manuf. 2021;37:101639.
Bhattacharyya A, Priya VNK, Kim JH, Khatun MR, Nagarajan R, Noh I. Nanodiamond enhanced mechanical and biological properties of extrudable gelatin hydrogel cross-linked with tannic acid and ferrous sulphate. Biomater Res. 2022;26:37.
Khatun MR, Bhattacharyya A, Taheri S, Ham Hw, Kim H, Chang SH, et al. High molecular weight fucoidan loading into and release from hyaluronate-based prefabricated hydrogel and its nanogel particles controlled by variable pitch and differential extensional shear technology of advanced twin screw‐based system. Adv Mater Technol. 2022;8:2201478.
Lee J, Kim KE, Bang S, Noh I, Lee C. A desktop multi-material 3D bio-printing system with open-source hardware and software. Int J Precis Eng Manuf. 2017;18:605–12.
Pillai MM, Tran HN, Sathishkumar G, Manimekalai K, Yoon J, Lim D, et al. Symbiotic culture of nanocellulose pellicle: a potential matrix for 3D bioprinting. Mater Sci Eng C Mater Biol Appl. 2021;119:111552.
Janarthanan G, Lee S, Noh I. 3D Printing of bioinspired alginate-albumin based instant gel ink with electroconductivity and its expansion to direct four‐axis printing of hollow porous tubular constructs without supporting materials. Adv Funct Mater. 2021;31:2104441.
Das D, Pham TTH, Noh I. Characterizations of hyaluronate-based terpolymeric hydrogel synthesized via free radical polymerization mechanism for biomedical applications. Colloids Surf B Biointerfaces. 2018;170:64–75.
Bui X, Dang T. Bioactive glass 58S prepared using an innovation sol-gel process. Process Appl Ceram. 2019;13:98–103.
Madsen IC, Scarlett NVY, Kern A. Description and survey of methodologies for the determination of amorphous content via X-ray powder diffraction. Z Kristallogr. 2011;226:944–55.
Bhattacharyya A, Janarthanan G, Kim T, Taheri S, Shin J, Kim J, et al. Modulation of bioactive calcium phosphate micro/nanoparticle size and shape during in situ synthesis of photo-crosslinkable gelatin methacryloyl based nanocomposite hydrogels for 3D bioprinting and tissue engineering. Biomater Res. 2022;26:54.
Zarrintaj P, Manouchehri S, Ahmadi Z, Saeb MR, Urbanska AM, Kaplan DL, et al. Agarose-based biomaterials for tissue engineering. Carbohydr Polym. 2018;187:66–84.
Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–22.
Killion JA, Kehoe S, Geever LM, Devine DM, Sheehan E, Boyd D, et al. Hydrogel/bioactive glass composites for bone regeneration applications: synthesis and characterisation. Mater Sci Eng C Mater Biol Appl. 2013;33:4203–12.
Kotuniak R, Strampraad MJF, Bossak-Ahmad K, Wawrzyniak UE, Ufnalska I, Hagedoorn PL, et al. Key Intermediate Species reveal the copper(II)-Exchange pathway in Biorelevant ATCUN/NTS Complexes. Angew Chem Int Ed Engl. 2020;59:11234–9.
Acknowledgements
Authors acknowledge the support provided by National Research Foundation of Korea, Seoul National University of Science and Technology and PSG Institutions to conduct the research. We also thank Shiva Taheri and HyeRim Cho for their in vitro cell culture assistance. National Research Foundation of Korea (Brain Pool Fellowship 2021H1D3A2A02044451 and 2022R1A2C2006341) and Green Convergence Technology Specialized Graduate Program through the Korea Environmental Industry and Technology Institute (KEITI) funded by the Ministry of Environment (MOE).
Author information
Authors and Affiliations
Contributions
AB designed all the experiments, analyzed the data, and wrote the manuscript, MRK 3D printed samples, SN optimized the gel compositions, RN characterized the gel, IN supervised the research. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no competing interest.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 11621.9 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhattacharyya, A., Khatun, M.R., Narmatha, S. et al. Modulation of 3D Bioprintability in Polysaccharide Bioink by Bioglass Nanoparticles and Multiple Metal Ions for Tissue Engineering. Tissue Eng Regen Med 21, 261–275 (2024). https://doi.org/10.1007/s13770-023-00605-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00605-1