Abstract
Background:
Extracellular vesicles (EVs) derived from plants have emerged as potential candidates for cosmetic and therapeutic applications. In this study, we isolated EVs from Aloe vera peels (A-EVs) and investigated the antioxidant and wound healing potential of A-EVs.
Methods:
A-EVs were isolated by ultracentrifugation and tangential flow filtration and were characterized using transmission electron microscopy, nanoparticle tracking analysis. The cytotoxicity and cellular uptake of A-EVs were investigated by WST-1 assay and flow cytometry. The antioxidant effect of A-EVs was evaluated by superoxide dismutase (SOD) activity assay and cellular antioxidant activity (CAA) assay. The wound healing potential was assessed by in vitro scratch assay using human keratinocytes (HaCaT) and fibroblasts (HDF). The expression of nuclear factor erythroid 2–related factor 2 (Nrf2) and their associated genes was analyzed by quantitative RT-PCR.
Results:
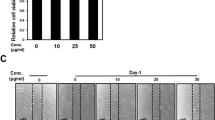
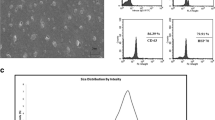
A-EVs displayed a round shape and had diameters from 50 to 200 nm. A-EVs showed good cytocompatibility on human skin cells and were internalized into HaCaT cells via clathrin-, caveolae-mediated endocytosis, and membrane fusion. The SOD activity and CAA assays exhibited that A-EVs had antioxidant activity and reduced intracellular ROS levels in H2O2-treated HaCaT cells in a dose-dependent manner. A scratch assay showed that A-EVs enhanced the migration ability of HaCaT and HDF. Moreover, A-EVs significantly upregulated the mRNA expression of Nrf2, HO-1, CAT, and SOD genes in H2O2-treated HaCaT cells. Our findings reveal that A-EVs could activate the antioxidant defense mechanisms and wound healing process via the Nrf2 activation.
Conclusion:
Overall results suggest that the A-EVs are promising as a potential agent for skin regeneration.








Similar content being viewed by others
References
Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;5:35–43.
Takeo M, Lee W, Ito M. Wound healing and skin regeneration. Cold Spring Harb Perspect Med. 2015;5:a023267.
Blanpain C. Skin regeneration and repair. Nature. 2010;464:686–7.
Liu Z, Ren Z, Zhang J, Chuang CC, Kandaswamy E, Zhou T, et al. Role of ROS and nutritional antioxidants in human diseases. Front Physiol. 2018;9:477.
Kim W, Lee S, Seo D, Kim D, Kim K, Kim E, et al. Cellular stress responses in radiotherapy. Cells. 2019;8:1105.
Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev 2013;2013:956792.
Brieger K, Schiavone S, Miller FJ, Krause KH. Reactive oxygen species: from health to disease. Swiss Med Wkly. 2012;142:w13659.
Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. Biomed Res Int. 2012;2012:936486.
Habtemariam S. Modulation of reactive oxygen species in health and disease. Antioxidants (Basel). 2019;8:513.
Flora SJS. Role of free radicals and antioxidants in health and disease. Cell Mol Biol (Noisy-le-grand). 2007;53:1–2.
Chen Q, Wang Q, Zhu J, Xiao Q, Zhang L. Reactive oxygen species: key regulators in vascular health and diseases. Br J Pharmacol. 2018;175:1279–92.
Hultqvist M, Olsson LM, Gelderman KA, Holmadahl R. The protective role of ROS in autoimmune disease. Trends Immunol. 2009;30:201–8.
Bardaweel SK, Gul M, Alzweiri M, Ishaqat A, ALSalamat HA, Bashatwah RM. Reactive oxygen Species: the dual role in physiological and pathological conditions of the human body. Eurasian J Med 2018;50:193–201
Bergamini CM, Gambetti S, Dondi A, Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des. 2004;10:1611–26.
Cabiscol E, Tamarit J, Ros J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol. 2000;3:3–8.
Ha HJ, Hwang IA, Park JH, Lee HB. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res Clin Pract. 2008;82:S42–5.
Singh A, Kukreti R, Saso L, Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules. 2019;24:1583.
Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11.
Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19:42.
Jacinto TA, Meireles GS, Dias AT, Aires R, Porto ML, et al. Increased ROS production and DNA damage in monocytes are biomarkers of aging and atherosclerosis. Biol Res. 2018;51:33.
Foo NP, Lin SH, Lee YH, Wu MJ, Wang YJ. α-Lipoic acid inhibits liver fibrosis through the attenuation of ROS-triggered signaling in hepatic stellate cells activated by PDGF and TGF-β. Toxicology. 2011;282:39–46.
Tian Z, Chen Y, Yao N, Hu C, Wu Y, Guo D, et al. Role of mitophagy regulation by ROS in hepatic stellate cells during acute liver failure. Am J Physiol Gastrointest Liver Physiol. 2018;315:G374–84.
Ma X, Dang C, Kang H, Dai Z, Lin S, Guan H, et al. Saikosaponin-D reduces cisplatin-induced nephrotoxicity by repressing ROS-mediated activation of MAPK and NF-kappaB signalling pathways. Int Immunopharmacol. 2015;28:399–408.
Li Y, Li X, Wong YS, Chen T, Zhang H, Liu C, et al. The reversal of cisplatin-induced nephrotoxicity by selenium nanoparticles functionalized with 11-mercapto-1-undecanol by inhibition of ROS-mediated apoptosis. Biomaterials. 2011;32:9068–76.
Blaser H, Dostert C, Mak TW, Brenner D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016;26:249–61.
Ambrozova N, Ulrichova J, Galandakova A. Models for the study of skin wound healing. The role of Nrf2 and NF-kB. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2017;161:1–13
Pedersen TX, Leethanakul C, Patel V, Mitola D, Lund LR, Danø K, et al. Laser capture microdissection-based in vivo genomic profiling of wound keratinocytes identifies similarities and differences to squamous cell carcinoma. Oncogene. 2003;22:3964–76.
Braun S, Hanselmann C, Gassmann MG, auf dem Keller U, Born-Berclaz C, Chan K, et al. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol Cell Biol. 2002;22:5492–505.
Barku VYA. Wound healing: contributions from plant secondary metabolite antioxidants. In: Dogan KH, editor. Wound healing-current perspectives. London: IntechOpen; 2019. p. 49–63.
Schäfer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res. 2008;58:165–71.
Long M, Rojo de la Vega M, Wen Q, Bharara M, Jiang T, Zhang R, et al. An essential role of Nrf2 in diabetic wound healing. Diabetes. 2016;65:780–93.
Caddeo C, Manca ML, Peris JE, Usach I, Diez-Sales O, Matos M, et al. Tocopherol-loaded transfersomes: in vitro antioxidant activity and efficacy in skin regeneration. Int J Pharm. 2018;551:34–41.
Fitzmaurice SD, Sivamani RK, Isseroff RR. Antioxidant therapies for wound healing: a clinical guide to currently commercially available products. Skin Pharmacol Physiol. 2011;24:113–26.
Xu DP, Li Y, Meng X, Zhou T, Zhou Y, Zheng J, et al. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int J Mol Sci. 2017;18:96.
Petruk G, Del Giudice R, Rigano MM, Monti DM. Antioxidants from plants protect against skin photoaging. Oxid Med Cell Longev. 2018;2018:1454936.
Rodrigues LLO, de Oliveira ACL, Tabrez S, Shakil S, Khan MI, Asghar MN, et al. Mutagenic, antioxidant and wound healing properties of Aloe vera. J Ethnopharmacol. 2018;227:191–7.
Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012;2012:1–26.
Yuan L, Duan X, Zhang R, Zhang Y, Qu M. Aloe polysaccharide protects skin cells from UVB irradiation through Keap1/Nrf2/ARE signal pathway. J Dermatolog Treat. 2020;31:300–8.
Xu Q, Fan Y, Loor JJ, Liang Y, Lv H, Sun X, et al. Aloin protects mice from diet-induced non-alcoholic steatohepatitis via activation of Nrf2/HO-1 signaling. Food Funct. 2021;12:696–705.
Ceravolo I, Mannino F, Irrera N, Squadrito F, Altavilla D, Ceravolo G, et al. Health potential of Aloe vera against oxidative stress induced corneal damage: an “in vitro” study. Antioxidants (Basel). 2021;10:318.
Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013;21:1345–57.
Lee R, Ko HJ, Kim K, Sohn Y, Min SY, Kim JA, et al. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J Extracell Vesicles. 2020;9:1703480.
Zhang M, Viennois E, Xu C, Merlin D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers. 2016;4:e1134415.
Mu J, Zhuang X, Wang Q, Jiang H, Deng ZB, Wang B, et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58:1561–73.
Dad HA, Gu TW, Zhu AQ, Huang LQ, Peng LH. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021;29:13–31.
Zhuang X, Deng ZB, Mu J, Zhang L, Yan J, Miller D, et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4:28713.
Kahroba H, Davatgaran-Taghipour Y. Exosomal Nrf2: From anti-oxidant and anti-inflammation response to wound healing and tissue regeneration in aged-related diseases. Biochimie. 2020;171–2:103–9.
Zhang M, Viennois E, Prasad M, Zhang Y, Wang L, Zhang Z et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–40.
Xiao J, Feng S, Wang X, Long K, Luo Y, Wang Y, et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ. 2018;6:e5186.
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
Guo L, Zhang Y, Wei R, Zhang X, Wang C, Feng M. Proinflammatory macrophage-derived microvesicles exhibit tumor tropism dependent on CCL2/CCR2 signaling axis and promote drug delivery via SNARE-mediated membrane fusion. Theranostics. 2020;10:6581–98.
Wolfe RH, Liu KL. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods and dietary supplements. J Agric Food Chem. 2007;55:8896–907.
Yang C, Zhang M, Merlin D. Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J Mater Chem B. 2018;6:1312–21.
Quispe C, Villalobos M, Bórquez J, Simirgiotis M. Chemical composition and antioxidant activity of Aloe vera from the pica oasis (Tarapacá, Chile) by UHPLC-Q/Orbitrap/MS/MS. J Chem. 2018;2018:6123850.
Busatto S, Vilanilam G, Ticer T, Lin WL, Dickson DW, Shapiro S, et al. Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells. 2018;7:273.
Hüppi PS. The role of oxygen in health and disease–a series of reviews. Pediatr Res. 2009;65:261–8.
Francisqueti-Ferron FV, Ferron AJT, Garcia JL, Silva CCVA, Costa M, Gregolin CS, et al. Basic concepts on the role of nuclear factor erythroid-derived 2-like 2 (Nrf2) in age-related diseases. Int J Mol Sci. 2019;20:3208.
Senger DR, Cao S. Diabetic wound healing and activation of Nrf2 by herbal medicine. J Nat Sci. 2016;2:e247.
Schanuel FS, Saguie BO, Monte-Alto-Costa A. Olive oil promotes wound healing of mice pressure injuries through NOS-2 and Nrf2. Appl Physiol Nutr Metab. 2019;44:1199–208.
Muniandy K, Gothai S, Tan WS, Kumar SS, Mohd Esa N, Chandramohan G, et al. In vitro wound healing potential of stem extract of Alternanthera sessilis. Evid Based Complement Alternat Med. 2018;2018:3142073.
Jorge MP, Madjarof C, Gois Ruiz AL, Fernandes AT, Ferreira Rodrigues RA, de Oliveira Sousa IM, et al. Evaluation of wound healing properties of Arrabidaea chica verlot extract. J Ethnopharmacol. 2008;118:361–6.
Dissemond J, Goos M, Wagner SN. The role of oxidative stress in the pathogenesis and therapy of chronic wounds. Hautarzt. 2002;53:718–23.
Arunachalam K, Parimelazhagan T. Anti-inflammatory, wound healing and in-vivo antioxidant properties of the leaves of Ficus amplissima Smith. J Ethnopharmacol. 2013;145:139–45.
Richter-Landsberg C, Vollgraf U. Mode of cell injury and death after hydrogen peroxide exposure in cultured oligodendroglia cells. Exp Cell Res. 1998;244:218–29.
Uhl L, Geretel A, Chabalier M, Dukan S. Hydrogen peroxide induced cell death: One or two modes of action? Heliyon. 2015;1:e00049.
Horibe S, Tanahashi T, Kawauchi S, Murakami Y, Rikitake Y. Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer. 2018;18:47.
Pan H, Wang H, Zhu L, Mao L, Qiao L, Su X. The role of Nrf2 in migration and invasion of human glioma Cell U251. World Neurosurg. 2013;80:363–70.
Acknowledgement
This research was supported and funded by Exostemtech Inc.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Yong Woo Cho are stockholders of Exostemtech Inc. Min Kang Kim, Young Chan Choi, Seung Hee Cho, and Ji Suk Choi are employees of Exostemtech Inc.
Ethical statement
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, M.K., Choi, Y.C., Cho, S.H. et al. The Antioxidant Effect of Small Extracellular Vesicles Derived from Aloe vera Peels for Wound Healing. Tissue Eng Regen Med 18, 561–571 (2021). https://doi.org/10.1007/s13770-021-00367-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-021-00367-8