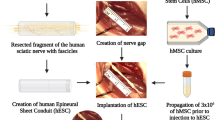
Abstract
BACKGROUND:
Centella asiatica (L.) is a plant with neuroprotective and neuroregenerative properties; however, its effects on the neurodifferentiation of mesenchymal stem cells (MSCs) and on peripheral nerve injury are poorly explored. This study aimed to investigate the effects of C. asiatica (L.)-neurodifferentiated MSCs on the regeneration of peripheral nerve in a critical-size defect animal model.
METHODS:
Nerve conduit was developed using decellularised artery seeded with C. asiatica-neurodifferentiated MSCs (ndMSCs). A 1.5 cm sciatic nerve injury in Sprague–Dawley rat was bridged with reversed autograft (RA) (n = 3, the gold standard treatment), MSC-seeded conduit (MC) (n = 4) or ndMSC-seeded conduit (NC) (n = 4). Pinch test and nerve conduction study were performed every 2 weeks for a total of 12 weeks. At the 12th week, the conduits were examined by histology and transmission electron microscopy.
RESULTS:
NC implantation improved the rats’ sensory sensitivity in a similar manner to RA. At the 12th week, nerve conduction velocity was the highest in NC compared with that of RA and MC. Axonal regeneration was enhanced in NC and RA as shown by the expression of myelin basic protein (MBP). The average number of myelinated axons was significantly higher in NC than in MC but significantly lower than in RA. The myelin sheath thickness was higher in NC than in MC but lower than in RA.
CONCLUSION:
NC showed promising effects on nerve regeneration and functional restoration similar to those of RA. These findings revealed the neuroregenerative properties of C. asiatica and its potential as an alternative strategy for the treatment of critical size nerve defect.








Similar content being viewed by others
References
Kaizawa Y, Kakinoki R, Ikeguchi R, Ohta S, Noguchi T, Takeuchi H, et al. A nerve conduit containing a vascular bundle and implanted with bone marrow stromal cells and decellularized allogenic nerve matrix. Cell Transplant. 2017;26:215–28.
Hoffman PN, Lasek RJ. Axonal transport of the cytoskeleton in regenerating motor neurons: constancy and change. Brain Res. 1980;202:317–33.
Ray WZ, Mackinnon SE. Management of nerve gaps: autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp Neurol. 2010;223:77–85.
Gordon T, Sulaiman O, Boyd JG. Experimental strategies to promote functional recovery after peripheral nerve injuries. J Peripher Nerv Syst. 2003;8:236–50.
Gu X, Ding F, Williams DF. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials. 2014;35:6143–56.
Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113:1701–10.
Jonsson S, Wiberg R, McGrath AM, Novikov LN, Wiberg M, Novikova LN, et al. Effect of delayed peripheral nerve repair on nerve regeneration, Schwann cell function and target muscle recovery. PLoS One. 2013;8:e56484.
Kwong KW, Sivakumar T, Wong WK. Intein mediated hyper-production of authentic human basic fibroblast growth factor in Escherichia coli. Sci Rep. 2016;6:33948.
Li D, Yuan T, Zhang X, Xiao Y, Fan Y, Wang R. Icariin: a potential promoting compound for cartilage tissue engineering. Osteoarthritis Cartilage 2012;20:1647–56.
Raghavan RN, Vignesh G, Kumar BS, Selvaraj R, Dare BS. Phytochemicals: do they hold the future in stem cell differentiation. Int J Res Pharma. 2015;6:379–81.
Wakao S, Matsuse D, Dezawa M. Mesenchymal stem cells as a source of Schwann cells: their anticipated use in peripheral nerve regeneration. Cells Tissues Organs. 2014;200:31–41.
Jadalannagari S, Aljitawi OS. Ectodermal differentiation of wharton’s jelly mesenchymal stem cells for tissue engineering and regenerative medicine applications. Tissue Eng Part B Rev. 2015;21:314–22.
Lokanathan Y, Omar N, Ahmad Puzi NN, Saim A, Hj Idrus R. Recent updates in neuroprotective and neuroregenerative potential of Centella asiatica. Malays J Med Sci. 2016;23:4–14.
Soumyanath A, Zhong YP, Gold SA, Yu X, Koop DR, Bourdette D, et al. Centella asiatica accelerates nerve regeneration upon oral administration and contains multiple active fractions increasing neurite elongation in-vitro. J Pharm Pharmacol. 2005;57:1221–9.
Norazzila O, Yogeswaran L, Mohd Razi ZR, Bt Haji Idrus R. The effects of Centella asiatica (L.) Urban on neural differentiation of human mesenchymal stem cells in vitro. BMC Complement Altern Med. 2019;19:167.
Hussin HM, Idrus RH, Lokanathan Y. Development of nerve conduit using decellularized human umbilical cord artery seeded with Centella asiatica induced-neurodifferentiated human mesenchymal stem cell. Sains Malays. 2018;47:2789–98.
Leow SN, Luu CD, Hairul Nizam MH, Mok PL, Ruhaslizan R, Wong HS, et al. Safety and efficacy of human Wharton’s Jelly-derived mesenchymal stem cells therapy for retinal degeneration. PLoS One. 2015;10:e0128973.
Wang Y, Qi F, Zhu S, Ye Z, Ma T, Hu X, et al. A synthetic oxygen carrier in fibrin matrices promotes sciatic nerve regeneration in rats. Acta Biomater. 2013;9:7248–63.
Bongso A, Fong CY. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton’s jelly of the human umbilical cord. Stem Cell Rev Rep. 2013;9:226–40.
Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, et al. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells. 2008;26:2865–74.
Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60.
Yurie H, Ikeguchi R, Aoyama T, Kaizawa Y, Tajino J, Ito A, et al. The efficacy of a scaffold-free Bio 3D conduit developed from human fibroblasts on peripheral nerve regeneration in a rat sciatic nerve model. PLoS One. 2017;12:e0171448.
Lokanathan Y, Ng MH, Hasan S, Ali A, Mahmod M, Htwe O, et al. Olfactory ensheathing cells seeded muscle-stuffed vein as nerve conduit for peripheral nerve repair: a nerve conduction study. J Biosci Bioeng. 2014;118:231–4.
Costa MP, Teixeira NH, Longo MV, Gemperli R, Costa HJ. Combined polyglycolic acid tube and autografting versus autografting or polyglycolic acid tube alone A comparative study of peripheral nerve regeneration in rats. Acta Cir Bras. 2015;30:46–53.
Carr MM, Best TJ, Mackinnon SE, Evans PJ. Strain differences in autotomy in rats undergoing sciatic nerve transection or repair. Ann Plast Surg. 1992;28:538–44.
Klusáková I, Dubový P. Experimental models of peripheral neuropathic pain based on traumatic nerve injuries–an anatomical perspective. Ann Anat. 2009;191:248–59.
Ao Q, Fung CK, Tsui AY, Cai S, Zuo HC, Chan YS, et al. The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells. Biomaterials. 2011;32:787–96.
Martini R, Mohajeri MH, Kasper S, Giese KP, Schachner M. Mice doubly deficient in the genes for P0 and myelin basic protein show that both proteins contribute to the formation of the major dense line in peripheral nerve myelin. J Neurosci. 1995;15:4488–95.
Taniuchi M, Clark HB, Johnson EM Jr. Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci U S A. 1986;83:4094–8.
Tohill M, Mantovani C, Wiberg M, Terenghi G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 2004;362:200–3.
Michetti F, Corvino V, Geloso MC, Lattanzi W, Bernardini C, Serpero L, et al. The S100B protein in biological fluids: more than a lifelong biomarker of brain distress. J Neurochem. 2012;120:644–59.
Haastert K, Mauritz C, Matthies C, Grothe C. Autologous adult human Schwann cells genetically modified to provide alternative cellular transplants in peripheral nerve regeneration. J Neurosurg. 2006;104:778–86.
Novikova LN, Pettersson J, Brohlin M, Wiberg M, Novikov LN. Biodegradable poly-β-hydroxybutyrate scaffold seeded with Schwann cells to promote spinal cord repair. Biomaterials. 2008;29:1198–206.
Dezawa M, Takahashi I, Esaki M, Takano M, Sawada H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur J Neurosci. 2001;14:1771–6.
Tedesco D, Haragsim L. Cyclosporine: a review. J Transplant. 2012;2012:230386.
Brohlin M, Mahay D, Novikov LN, Terenghi G, Wiberg M, Shawcross SG, et al. Characterisation of human mesenchymal stem cells following differentiation into Schwann cell-like cells. Neurosci Res. 2009;64:41–9.
Koning M, Harmsen MC, van Luyn MJ, Werker PM. Current opportunities and challenges in skeletal muscle tissue engineering. J Tissue Eng Regen Med. 2009;3:407–15.
Acknowledgements
This study was funded by the Ministry of Agriculture and Agro-based Industry Malaysia under NKEA Research Grant Scheme (NRGS) (Project Code: NH 1014 D048) and Universiti Kebangsaan Malaysia (Project Code: FF-2017-175 and GUP-2017-007). The author would like to thank Prof. Dr. Mohd Ilham Adenan from Atta-ur-Rahman Institute for Natural Product Discovery, Universiti Teknologi MARA, Malaysia, who kindly provided C. asiatica extract for use in this experiment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
The animal study was approved by Universiti Kebangsaan Malaysia (UKM) Animal Ethics Committee (TEC/PP/2016/YOGESWARAN/18-MAY/747-MAY-2016-FEB.-2018). For the isolation of human mesenchymal stem cell (hMSCs), the written informed consents were obtained from healthy women who delivered full-term infants (38–40 weeks) by elective caesarian delivery prior to the collection of umbilical cord samples. The usage of human umbilical cord samples from consenting patients in this study was approved by the Universiti Kebangsaan Malaysia Research Ethics Committee (FF-2015-175).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hussin, H.M., Lawi, M.M., Haflah, N.H.M. et al. Centella asiatica (L.)-Neurodifferentiated Mesenchymal Stem Cells Promote the Regeneration of Peripheral Nerve. Tissue Eng Regen Med 17, 237–251 (2020). https://doi.org/10.1007/s13770-019-00235-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-019-00235-6