Abstract
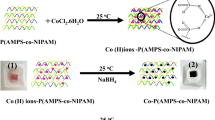
This work describes the synthesis of poly(acrylic acid) microgels and fabrication of magnetic cobalt nanoparticles in the prepared microgels. Cobalt nanoparticles were fabricated by loading the cobalt (II) ions in microgels from aqueous solution and their subsequent reduction with sodium borohydride (NaBH4). Bare and composite microgels were characterized by Fourier transform infrared spectroscopy, scanning electron microscopy and transmission electron microscopy. The catalytic properties of the prepared microgel composites were investigated by using them as catalyst for the reduction of 4-nitrophenol and methylene blue. The effect of temperature and catalyst dose on the rate of reduction of these toxic pollutants was investigated. The reusability of prepared catalysts was also studied for the five consecutive cycles, and an increase in catalytic activity was observed after every cycle. The prepared bare and magnetic microgels were found as very effective adsorbent for the removal of methylene blue from aqueous medium. Very rapid adsorption rate was found for the removal of methylene as its 100 mg was adsorbed on per gram of dried hydrogels in about 25 min. The effects of different parameters like amount of adsorbate and concentration of adsorbent on the adsorption process were studied. Langmuir, Freundlich and Temkin adsorption isotherms were applied, and it was found that adsorption of MB follows Freundlich model better than others. Furthermore, pseudo-first-order and pseudo-second-order kinetic models were also applied and adsorption of MB was found to abide by pseudo-second-order kinetics.








Similar content being viewed by others
References
Ahmad T, Rafatullah M, Ghazali A, Sulaiman O, Hashim R, Ahmad A (2010) Removal of pesticides from water and wastewater by different adsorbents: a review. J Environ Sci Heal C 28:231–271
Ajmal M, Siddiq M, Al-Lohedan H, Sahiner N (2014) Highly versatile p (MAc)–M (M: Cu Co, Ni) microgel composite catalyst for individual and simultaneous catalytic reduction of nitro compounds and dyes. RSC Adv 4:59562–59570
Ajmal M, Demirci S, Siddiq M, Aktas N, Sahiner N (2015a) Betaine microgel preparation from 2-(methacryloyloxy) ethyl] dimethyl (3-sulfopropyl) ammonium hydroxide and its use as a catalyst system. Colloid Surf A 486:29–37
Ajmal M, Siddiq M, Aktas N, Sahiner N (2015b) Magnetic Co–Fe bimetallic nanoparticle containing modifiable microgels for the removal of heavy metal ions, organic dyes and herbicides from aqueous media. RSC Adv 5:43873–43884
Ajmal M, Demirci S, Siddiq M, Aktas N, Sahiner N (2016) Simultaneous catalytic degradation/reduction of multiple organic compounds by modifiable p (methacrylic acid-co-acrylonitrile)–M (M: Cu, Co) microgel catalyst composites. New J Chem 40:1485–1496
Al-Husseiny HA (2014) Adsorption of methylene blue dye using low cost adsorbent of sawdust: batch and continues studies. J Babylon Univ/Eng Sci 22:296–310
Ayad MM, El-Nasr AA (2010) Adsorption of cationic dye (methylene blue) from water using polyaniline nanotubes base. J Phys Chem C 114:14377–14383
Böhmer U, Franke F, Morgenschweis K, Bieber T, Reschetilowski W (2000) Enantioselective hydrogenation of ethyl pyruvate: long-term performance of chirally modified Pt/zeolite catalysts. Catal Today 60:167–173
Butun S, Sahiner N (2011) A versatile hydrogel template for metal nano particle preparation and their use in catalysis. Polymer 52:4834–4840
Dai R, Chen J, Lin J, Xiao S, Chen S, Deng Y (2009) Reduction of nitro phenols using nitroreductase from E. coli in the presence of NADH. J Hazard Mater 170:141–143
de Santa Maria LC, Amorim MC, Aguiar MR, Guimarães PIC, Costa MA, de Aguiar AP, Rezende PR, de Carvalho MS, Barbosa FG, Andrade JM (2001) Chemical modification of cross-linked resin based on acrylonitrile for anchoring metal ions. React Funct Polym 49:133–143
Du X, He J, Zhu J, Sun L, An S (2012) Ag-deposited silica-coated Fe3O4 magnetic nanoparticles catalyzed reduction of p-nitrophenol. Appl Surf Sci 258:2717–2723
Feng Y, Schmidt A, Weiss R (1996) Compatibilization of polymer blends by complexation. 1. Spectroscopic characterization of ion-amide interactions in ionomer/polyamide blends. Macromolecules 29:3909–3917
Gu S, Wunder S, Lu Y, Ballauf M (2014) Kinetic analysis of the catalytic reduction of 4-nitrophenol by metallic nanoparticles. J Phys Chem C 2014(118):18618–18625
Hayakawa K, Yoshimura T, Esumi K (2003) Preparation of gold-dendrimer nanocomposites by laser irradiation and their catalytic reduction of 4-nitrophenol. Langmuir 19:5517–5521
Hernández R, Mijangos C (2009) In situ synthesis of magnetic iron oxide nanoparticles in thermally responsive alginate-poly (N-isopropylacrylamide) semi-interpenetrating polymer networks. Macromol Rapid Commun 30:176–181
Ho Y, Gordon M (1999) Pseudo-second order model for sorption processes. Process Biochem 5:451–465
Khan A, El-Toni AM, Alrokayan S, Alsalhi M, Alhoshan M, Aldwayyan AS (2011) Microwave-assisted synthesis of silver nanoparticles using poly-N-isopropylacrylamide/acrylic acid microgel particles. Colloid Surf A 377:356–360
Mak S-Y, Chen DH (2004) Fast adsorption of methylene blue on polyacrylic acid-bound iron oxide magnetic nanoparticles. Dyes Pigments 61:93–98
Megharaj M, Pearson H, Venkateswarlu K (1991) Toxicity of phenol and three nitrophenols towards growth and metabolic activities of Nostoc linckia, isolated from soil. Arch Environ Contam Toxicol 21:578–584
Ozay O, Ekici S, Baran Y, Aktas N, Sahiner N (2009) Removal of toxic metal ions with magnetic hydrogels. Water Res 43:4403–4411
Ozay O, Ekici S, Baran Y, Kubilay S, Aktas N, Sahiner N (2010) Utilization of magnetic hydrogels in the separation of toxic metal ions from aqueous environment. Desalination 260:57–64
Ozay O, Inger E, Aktas N, Sahiner N (2011) Hydrogen production from ammonia borane via hydrogel template synthesized Cu, Ni, Co composites. Int J Hydrog Energy 36:8209–8216
Robello DR, Mis MR, Nair M (2015) Micron‐sized membrane reactors: multicompartment semipermeable polymer particles containing palladium nanoparticles. J Appl Polym Sci 132. doi:10.1002/app.42021
Sahiner N, Ozay H, Ozay O, Aktas N (2010a) A soft hydrogel reactor for cobalt nanoparticle preparation and use in the reduction of nitrophenols. Appl Catal B 101:137–143
Sahiner N, Ozay H, Ozay O, Aktas N (2010b) New catalytic route: hydrogels as templates and reactors for in situ Ni nanoparticle synthesis and usage in the reduction of 2-and 4-nitrophenols. Appl Catal A 385:201–207
Shaoqing Y, Jun H, Jianlong W (2010) Radiation-induced catalytic degradation of p-nitrophenol (PNP) in the presence of TiO2 nanoparticles. Radiat Phys Chem 79:1039–1046
Shi et al (2013) Removal of methylene blue from aqueous solution by sorption on lignocellulose-g-poly(acrylic acid)/montmorillonite three-dimensional cross-linked polymeric network hydrogels. Polym Bull 70:1163–1179
Wang D, Xin HL, Yu Y, Wang H, Rus E, Muller DA, Abruña HD (2010) Pt-decorated PdCo@ Pd/C core–shell nanoparticles with enhanced stability and electrocatalytic activity for the oxygen reduction reaction. J Am Chem Soc 132:17664–17666
Wu Y et al (2009) Adsorption of copper ions and methylene blue in a single and binary system on wheat straw. J Chem Eng Data 12:3229–3234
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interface Sci 209:172–184
Yang S-T, Chen S, Chang Y, Cao A, Liu Y, Wang H (2011) Removal of methylene blue from aqueous solution by graphene oxide. J Colloid Interface Sci 359:24–29
Zendehdel M, Barati A, Alikhani H, Hekmat A (2010) Removal of methylene blue dye from wastewater by adsorption onto semi-inpenetrating polymer network hydrogels composed of acrylamide and acrylic acid copolymer and polyvinyl alcohol. J Environ Health Sci Eng 7:431–436
Zhang Z, Kong J (2011) Novel magnetic Fe3O4@C nanoparticles as adsorbents for removal of organic dyes from aqueous solution. J Hazard Mater 193:325–329
Zhou et al (2011) Removal of methylene blue dyes from wastewater using cellulose-based super adsorbent hydrogels. Polym Eng Sci 51:2417–2424
Acknowledgements
The authors highly acknowledge financial support from Quaid-i-Azam University Islamabad, Pakistan, under University Research Fund 2015.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: M. Abbaspour.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bibi, F., Ajmal, M., Naseer, F. et al. Preparation of magnetic microgels for catalytic reduction of 4-nitrophenol and removal of methylene blue from aqueous medium. Int. J. Environ. Sci. Technol. 15, 863–874 (2018). https://doi.org/10.1007/s13762-017-1446-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1446-4