Abstract
The development of new biotechnology methods can solve public concerns especially on global warming and environmental pollution. This can be achieved by using integrated biorefinery bioprocesses to introduce biomass as a very best renewable source, to replace petroleum and to develop new bio-based products in an industrial scale. Genetic engineering of organisms and improving bioreactors is currently substantial approaches to increase biorefinery yields. The use of various feedstocks in new-generation biorefinery requires a flexible route with the lowest limitations. Biological approaches have attracted substantial interest in the conversion of biomass into biomaterial and biofuel. As a result of increase in valuable products due to the biomass conversion, the interest on biorefinery is higher than that of petroleum. Biotechnology is an integral aspect of new biology. Through genetic and metabolic engineering, bio-based products can be generated from living cells such as microalgae, bacteria and fungi. Greenhouse gas emission and CO2 can be released from the biomass feedstocks by chemical and thermochemical processes, while it can be reduced by increasing the use of biotechnological approach in biorefinery system. In order to manage our waste and at the same time produce valuable products, biotechnology approaches are very favorable and applicable than conventional methods. Collectively, the integration of biological routes with conventional routes can decrease operation cost by using waste materials, preventing environmental pollution, and, therefore, producing valuable by-products.
Similar content being viewed by others
Introduction
Climate change in today’s world demands environmental and economic care. Biotechnology approaches have provided new insights into replacement of petrochemical products and petroleum with bio-based and biofuels, by the conversion of biomass, which is abundant in nature (Gallezot 2012; Sarkar and Shimizu 2015). The composition of biomass as raw material provides a convincing explanation for the general use which is renewable, available and biodegradable (More et al. 2016).
Biotechnology is the discipline applicable in biological processes, and this includes any method that uses living cells or cellular components to make products or improve healthcare and agriculture, renew or modify products, or develop living cells for specific uses. High performance of chemical industry and growth in economy demands unrestricted energy resources, feasible large-scale techniques, and cheap feedstocks to produce valuable products (Stuart and El-Halwagi 2012).
Recently, genetic engineering of microorganisms is one of the most important interests in biotechnology to convert biomass to a series of bioproducts and biofuels (Stuart and El-Halwagi 2012). Incorporation of these techniques can result in reduction of greenhouse gas (GHG) emission, elevation of attempts to find any source of sustainable raw materials, and attainment of valuable food, feed and biofuel products.
Following the illustration of the genetic manipulation of living cells, many studies have focused on the role of engineered cells, enhancement of enzymes and chemical catalysts for production of bio-based materials and biofuels (Xue et al. 2015). Hence, in this review, we focus briefly on the capacities of microorganisms to participate in biorefinery system, new-generation biorefinery and municipal waste as an alternative precursor for feedstocks and bioenergy production (Ivanov et al. 2015).
History of biotechnology in biorefinery
In 1857, Louis Pasteur elucidated fermentation caused by microorganisms as one of the most important phenomena in biology. Incipient fermentation industry was born during the first part of the nineteenth century and soon two chemical product platform, ethanol and solvents such as butanol and acetone, became the main products. Evolution of fermentation industry continued with first marketing products, acid acetic and penicillin in 1923 and 1944, respectively (Willke and Vorlop 2004).
In the 1930s, the first- and second-generation biorefinery came to be established. Due to the industrialization of biotechnology and definition of modern fermentation, most of the bioproducts such as bioalcohols, bio-oil and biohydrogen were produced from agricultural biomass including corn, woods, sugar and wheat straw (Poggi-Varaldo et al. 2014; Stuart and El-Halwagi 2012).
During the decline of oil production after 1973, interest in bio-based fuel production has been soared in non-OPEC countries. The product of alternative fuel requires a technical approach. For this end, scientists suggested that the main energy supply could be from renewable resource. Metabolically engineered organisms have been developed to increase product yield. For example, ethanol production in the Zymomonas mobilis has been enhanced by metabolic engineering of xylose fermentation genes in 1995 (Zhang et al. 1995). In the last decades, a large number of organisms and their metabolic pathways, especially metabolic engineering, were also measured, such as Saccharomyces cerevisiae (S. cerevisiae), Escherichia coli (E. coli), Pichia stipitis and Klebsiella oxytoca (Walfridsson et al., 1996, Slininger et al., 1987).
The organic wastes as inexpensive raw materials have been introduced in recent decades to manage waste product, converting them to value-added product via biorefinery system. Recently, developing and using capable microorganisms to convert waste components have studied as a critical step in the waste management (Antoni et al. 2007; Leung et al. 2012).
In the past decades, biofuels such as bioethanol and biobutanol were discovered. Recently, bio hydrogen and bioelectricity are the two new bioenergy due to the new-generation biorefinery that split carbon components into high-energy forms using microorganisms (Kaparaju et al. 2009; Poggi-Varaldo et al. 2014).
Given the different capabilities of microorganisms in using biomass organic components, their manipulation and use is an interesting topic for biotechnology research. Utilization of living organisms were established and engineered, using aquatic organism like algae in the next generation of biorefinery system (Yen et al. 2013).
Microorganism: a micro manufactory in biorefinery
Fermentation of carbohydrates by living cells like microorganisms, especially their cellular metabolic utilization and enzymatic properties, provided new insights into biorefinery bioproducts (Alper and Stephanopoulos 2009). Microorganisms can utilize a variety of biomass feedstocks including carbohydrate and oil components. Although production of biomaterials and biofuels by thermochemical and chemical processes was practiced in the first generation, chemical processes are not environmentally friendly and their waste increased global pollution (Zou et al. 2016). To date, the development of new biotechnology methods was to minimize environmental effects of pollution and the best ways for recycling by-products from the outputs (Sanders et al. 2007; Zou et al. 2016).
Bioproducts produced from biomass are generally made via three main pathways: thermochemical, biochemical and physicochemical. Thermochemical platforms such as pyrolysis and gasification are a preliminary conversion pathway of biorefinery processes; it is characterized by high-temperature, pressure, and higher reaction rates (Haro et al. 2013). Due to acclivitous GHG emission and CO2 elicitation, biochemical routes include aerobic conversion, anaerobic decomposition or digestion, and anaerobic fermentation has exceedingly gained interest. The bioconversion strategy has environmentally advantages compare to chemical strategy such as the use of enzymatic hydrolysis, lower temperatures, lower CO2 formation and lower reaction rates (Sambusiti et al. 2016). Physicochemical routes mainly refer to the combination of physical and chemical processes, although chemical processes such as transesterification are more diversified than physical processes.
Thermochemical routes (such as steam reforming) have a major limitation; they seem to have a high economic performance in general, they need high temperature (T = 1200 °C) (Stuart and El-Halwagi 2012). Chemical processes are crucial steps in biorefinery and their energy requirement is less than thermochemical processes. Moreover, application of engineering cells and integrated chemicals and enzymatic pathway can increase the production of bio-based products. This process occurs in a biofermenter toward converting biomass content into bioproducts (Radakovits et al. 2010). Hence, utilization of microorganisms as both a sustainable and flexible alternative feedstocks, and micromanufactory in biorefinery system leads to (i) reductions of GHG emissions and production costs, (ii) the production of value-added biomaterials, new polymers and (iii) increased profitability.
Microalgae and its super energy components
Microalgae, a microscopically aquatic plant, have been the major feedstocks in the third generation milestone (Renuka et al. 2015). It possesses a great potential as a renewable carbon source and two major advantages in biorefinery: firstly, per unit area; microalgae can proliferate five times more than other energy plants, and secondly, in a poor proliferation area; microalgae can grow on an infertile land such as wastewater and saltwater (Tabatabaei et al. 2011).
In new-generation biorefinery, the utilization of microalgae, both biofuel production and wastewater treatment, has very interest for both biorefinery aspects. Therefore, sustainable product and harvest methods must eliminate the problem of water and biofuel supply (Pimentel and Patzek 2005; Yen et al. 2013; Christenson and Sims 2011).
The growth and sufficient lipid accumulation of microalgae depends on several factors including intensity of light source, temperature and nitrogen source availability (Chen et al. 2011; Ho et al. 2012). Recently, Ra et al. showed that the use of light-emitting diodes LEDs light as light stress could increase lipid accumulation of Picochlorum atomus, whereas the biomass production is increased by the exposing red light in the early stages and the green light could be remarkably enhanced lipid accumulation in second stage (Ra et al. 2016).
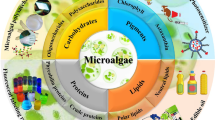
Carbohydrate is one of the important microalgae components, which is an energetic macromolecule and biological nutrients (Jang et al. 2012). In general, microalgae can remarkably accumulate carbohydrates content, approximately its make up higher than 50% of cell`s weight, by efficient photosynthetic process (Ho et al. 2012).
Extracellular polysaccharide (EPS) is another important microalgae biomass components. The isolation and purification of ESPs have a heavy important, because they can be split into bioactive molecules such as monosaccharides. One of the important cellular components of microalgae is glucose (monosaccharide) in biofuel production (Yen et al. 2013; John et al. 2011; Chochois et al. 2009). Recently, Goo et al. characterized and optimized acidic and enzymatic hydrolyses of Dunaliella tertiolecta EPS and introduced as a renewable biorefinery feedstock. Two hydrolytic methods, both acidic and enzymatic hydrolysis, can convert EPS into glucose (approximately 90% was recovered) in the optimized condition (Goo et al. 2013). In the last years, several high-value feedstocks of algae polysaccharides have been introduced which are capable of downstream application in many biorefinery aspects including: food, stabilizers, lubricants, cosmetics, and pharmacological agents (as shown in Fig. 1; Arad and Levy-Ontman 2010). There is, for instance, the polysaccharides content of Porphyridium sp. which has an antioxidant activity and can generate an antioxidative response against the radical oxygen species (ROS) stress. Hence, it has been introduced as an antiinflammatory drug against skin allergies (Tannin-Spitz et al. 2005).
Recent studies have found capable metabolic genes in biorefinery system, and these targets are developed to increase output. In green microalgae, a further in starch storage has been illustrated by the inhibition of hydrolytic and/or phosphorolytic mechanisms in starch degradation. In Arabidopsis thaliana (A. thaliana), the knockdown of α-amylase (AMY3) provides an ideal bioengineered approach for effective suppression of starch depolymerization (Smith et al. 2005).
The main component of microalgae cell storage is lipid, while is desired for biodiesel production (Fig. 2; Moazami et al. 2012). The majority of microalgae biofuel production using fatty acid chain containing 14-20 carbons are dedicated to biodiesel production from triacylglycerol (TAG) via the transesterification process (Yen et al. 2013). In the last decade, several studies have illustrated genetic transformation for ultimate cellular production of algae such as Chlorophyta and Rhodophyta (green and red algae, respectively; Gan et al. 2016). Therefore, two early-targeted transgenic processes of algae are both knockout and overexpression of metabolic enzymatically genes that can potentially accumulate fatty acid content for biofuel production. Furthermore, several mutation approaches illustrated that algae metabolic pathways are dependent on these. For instance, starchless mutant of Chlorella pyrenoidosa can invariably expand the polyunsaturated fatty acid synthesis (Radakovits et al. 2010). Several capable approaches to enhance microalgae lipid accumulation are shown in Table 1.

(Moazami et al. 2012)
Lipid-storage vacuole in microalgae cell
According to Fig. 1, other valuable microalgae bioproducts are pigments and proteins. Microalgae pigments such as Chlorophyll a are sensitive to both blue-violet wavelengths and orange-red, while excitation with these light wavelengths stimulates initiation electron donor in the photosynthetic electron-transport chain (Yen et al. 2013). Pheophorbide derived from chlorophyll and can be utilized in photodynamic therapy, with photosensitizer effects (Busch et al. 2009). In addition, photophorbide A (phA) as an antiglycation has been isolated from Capsosiphon fulvescens. Hong et al. proposed that phA can decrease the formation of furosine and Nε-(carboxymethyl) lysine; the two best biomarkers for initial and final glycation during diabetes, respectively. They proposed that it is a best therapeutic targets for the prevention of diabetes complications (Hong et al. 2016). Other microalgae bioproducts such as Astaxanthin and Phycobilins can be used as antioxidant agents and fluorescent labeling reagents, respectively (Yen et al. 2013).
Bacterial and fungal
Microbial-bioproducts have been generated by bioconversion of biomass as carbon source carried out by various organisms. While no microorganism naturally converts all organic content (such as carbohydrate, oil and protein) under biorefinery conditions, production occurs in more than one way. In order to cut down the excessive use of surplus metabolic way, S. cerevisiae (Sonderegger et al. 2004, Guo et al. 2016) and an E. coli (Cirino et al. 2006) strain have been engineered to xylose fermentation and other pentoses. Besides, utilization of photon energy, saccharide-free energy feedstock, has been demonstrated by photosynthetic cyanobacteria in bioreactors (Kunjapur and Eldridge 2010; Palmer and Brigham 2016; Sarkar and Shimizu 2015).
One of the most distinctive microbial biorefinery features is the use of Triacylglycerol (TAG) as a common lipid storage molecules for biofuel production by oleaginous, a microorganism that accumulates lipids (Jin et al. 2015).
Organisms such as insects, fungi and aquatic organisms naturally carry out chitin production. Evidence exists that, after cellulose, chitin is the second environmentally biopolymer in worldwide, an iterative structural by poly β (1-4) N-acetyl-d-glucosamine that more than 1010 metric tons are produced annually, especially in aquatic environment (Souza et al. 2011). Chitin is crucial members of feedstocks to microbial-TAG production; substantial marketing progress is required to facilitate and utilize the whole feedstocks (Zhang et al. 2011; Rinaudo 2006).
Several studies have demonstrated that Rhodococcus opacus (R. opacus) can naturally be used only as N-acetylglucosamine but not as d-glucosamine, solely as an carbon source (Palmer and Brigham 2016; Holder et al. 2011). Given the existence of high levels of both N-acetylglucosamine and d-glucosamine in natural chitosan and chitin, N-acetylglucosamine isolation via the chemical methods is more difficult than d-glucosamine isolation. To enhance production of TAG from N-acetylglucosamine, natural and engineered R. opacus PD630 can be used. However, recently, genetic recombinant R. opacus PD630 has demonstrated different capabilities of the synthesis and accumulation of TAG from xylose and cellobiose for 40 and 46% of CDW, respectively (Kurosawa et al. 2013; Hetzler and Steinbuchel 2013). Therefore, bioengineering enhance industrial bio production suitability and project profitability. Given the abundant existence of chitin in fishery waste, the utility of bioconversion can prevent the “food versus fuel” controversy.
One of the many organic acid products is citric acid in which produced by aerobic organisms due to the cellular respiration, and is continually used in food producing, pharmaceutical, nutraceutical, industrial and detergent additives (Zhang et al. 2015). Microbial fermentation is fundamentally process to organic acid production, especially in industrial scale. However, like the citric acid, some critically valuable chemical products such as oxalic acid can be produced naturally through bioconversion of biomass by fungi, for example, Aspergillus niger (A. niger). A. niger is a member of saprophytic species that can also exclusively produce citric acid and oxalic acid for commercial production (Lotfy et al. 2007; Kubicek et al. 1988). Glyoxylate dehydrogenase (GDH) and oxaloacetate hydrolase (OAH) enzymes are vital to biosynthesis of oxalic acid by microorganisms. However, in A. niger, GDH is inactive compared to OAH. When acetic acid and oxalic acid are produced in this pathway, the carbon–carbon bond cleavages, while it is present between the second and subsequent carbons of oxaloacetic acid, an especial target site for OAH-active site. In A. niger, the oxalic acid production by OAH is undoubtedly the main pathway, and OAH disruption results in oxalic acid defect. Depending on the overexpression of oahA gene (which is the only gene encoding OAH), the high yield of oxalic acid in A. niger has been achieved (Pedersen et al. 2000; Kobayashi et al. 2014).
Various biofuels have been produced by varieties of organisms, while next-generation biofuel like isoprenoid is synthesized by plants and algae with a limited production output and grows slowly with a large number of fatty acids. Isopentanol and isopentenol are derived from isoprenoid, and they are best substitute for gasoline. To enhance the rate of production, mevalonate (MVA) and deoxyxylulose-5-phosphate pathways were overexpressed in engineered S. cerevisiae and E. coli (Tsuruta et al., 2009, Westfall et al., 2012). Recently, to increase isoprene production, Yang et al. proposed a new MVA-mediated pathway in engineered E. coli (Yang et al., 2016a) followed by introducing two genes from Jeotgalicoccus specie and Elizabethkingia meningoseptica into E. coli, under flask and fed-batch fermentation condition, the isoprene products were 2.2 and 620 mg/L, respectively.
New-generation biorefinery
Nowadays, incineration and landfilling are still common processes in municipal solid waste (MSW) management of very undeveloped countries (Yan et al. 2016), which certainly creates high pollutant elements in the ambient environment and affects public health. Many hazardous elements such as Cd, Cu, Pb, Zn, Cr, Ni and Hg are released to environment due to incineration of MSW and also some hazardous elements such as wash oil and volatile organic compounds are released due to the landfilling (Slack et al. 2005; Stuart and KOSSON 1994; Karbassi et al. 2014). The climate change and the excessive product of pollution have led to the use of biological platform instead of chemical and thermochemical platforms. Biological platform is more advantageous because waste management result in value-added products.
Microbial anaerobic digestion is the best means of organic waste incineration. Recently, a new approach for utilization of municipal waste has been reported in which the waste is used as potential feedstocks for bio-based products in second-generation and new-generation biorefinery, called fourth-generation biorefinery (Stuart and El-Halwagi 2012).
Like the third generation, fourth-generation biorefinery can produce biofuel, but from new source of biomass with renewable capabilities. However, biofuel produced from traditional feedstocks such as corn, woods and microalgae has several limitations including economic performance, ecology footprint, geographical location and dependence on environment (Dutta et al. 2014). Given the availability and cost, the municipal waste is potential feedstocks that represent the most important component of organic and inorganic materials (Logroño et al. 2015; Kapdan and Kargi 2006). Several studies illustrated that the MSW contents (the carbohydrates, fats and proteins) are suitable feedstocks with several important factors (as shown in Fig. 3A) for bio-based products such as biomethane, biohydrogen, and bioelectricity generation due to biochemical and thermochemical energies embedded in them (Logroño et al. 2015; Poggi-Varaldo et al. 2014; Kapdan and Kargi 2006).
Municipal waste and biorefinery
Using organic waste to produce biofuel product is a better solution to both waste management and production of petroleum fuels. Municipal waste is undoubtedly significant renewable feedstocks to biorefinery production. In several studies, advanced biotechnology for bioproduction of MSW in the integrated biorefinery was reported. MSW mainly contains green waste (Biogenic), plastic and textile, mineral and other inorganic materials. By means of a normal bioproduction of bio-based MSW, the half of the mixed paper and approximately 40% of total green waste and woods can be used for biofuels production, under the optimal condition in biorefinery processes (Williams 2007).
The majority of MSW consists of organic components (Biowaste), such as carbohydrates, cellulose and fats. As illustrated in Fig. 4, food industry wastes are mainly new raw materials with a very high potential for biofuel and bioelectricity production, which can be generated during food supply chain (Conesa and Rey 2015; Logroño et al. 2015).
The primary studies established that the food wastes including municipal organic waste, sludge, food waste and agriculture waste can be used for volatile fatty acid (VFA) production, although the successes have been reported to a different extents (Singhania et al. 2013; Cai et al. 2004).
Biofuels production from industrial food waste is illustrated via fermentation of carbohydrates by S. cerevisiae (Hung et al. 2016). Therefore, S. Cerevisiae can produce ethanol via hydrolysis and fermentation. Concurrently, Yang et al. showed that for both methods the amount of ethanol production was 0.43 and 0.31 g/g, respectively. Furthermore, the cheap noodle waste was used and treated with amyloglucosidase and α-amylase. They also illustrated that an acceptable volume of ethanol was achieved (61.1 g/L) via simultaneous hydrolysis and fermentation by S. cerevisiae (Yang et al., 2014).
The most important bioproducts of restaurant wastes, especially cooking oil is biodiesel, produced via transesterification process (Babaki et al. 2017). Further experiments reported various sustainable methods for biodiesel production from cheap feedstocks such as canola oil, soybean oil, peanut oil and recently, cooking oil waste and vegetable oil waste which were utilized as very low-cost raw material. In biodiesel production using cooking oil, transesterification plays a major role, when organic R units exchanged between an ester and alcohol, it is dependent upon various parameters including reaction temperature and pressure. Lipozyme TL IM and Novozym 435 are two famous commercial enzymes (lipolytic enzyme) crucial in transesterification process (Seong et al. 2011; Lee et al. 2013).
Pretreatment typically drawbacks, environmentally and economical, arise during production of biofuel by using solvent and chemical agents in which is a necessary step in their production (Ravindran and Jaiswal 2016). Acidic agents or enzymes can be used for pretreatment. Although using enzymes is expensive, recently engineered fermentative organisms have improved its production (Howard et al. 2003; Gudynaite-Savitch and White 2016).
Lignocellulosic waste is one of the most important municipal wastes such as paper, it consists of cellulose and hemicellulose. Cellulolytic and hemicellulolytic enzymes were used for the lignocellulosic waste pre-treatment in biorefinery processes. In order to maximize production and minimize cost, Robins et al. introduced four enzymes into yeast, two devices were designed, one was the manganese peroxidase, and the other Aldo–Keto Reductase. They suggested that applying engineered yeast pretreatment for lignocellulosic biomass can reduce cost and improve biofuel yields (Robins and Rickus 2015).
During biomass microbial conversion, enzymatic hydrolysis occurs by various enzymes such as galactosidases, laccases, phytases and lipases, recently uses their cocktails (Fan et al. 2012; Schmid and Urlacher 2007; Narra et al. 2012; Chylenski et al. 2017). Enzyme is oldest value-added product that can be obtained from organisms such as S. cerevisiae, Aspergillus sp and Bacillus sp, and are naturally produced for utilization in biomass organic content while producing other high-value bioproducts such as biofuels (Panda et al. 2016; Annamalai et al. 2013).
Food industry waste such as kernels fruit, banana waste, orange bagasse and pineapple peel has been favored as low-cost raw materials for organism growth and is introduced as an ideal candidate for enzymatic production (Klein-Marcuschamer et al. 2012). The genetic engineering of fermentative organisms resulted in maximum production and profitability. Abundant production and high enzyme activity are two important parameters that result in genetic enhancements. In biotechnology concept, they are obtained by using media development and genetically efficient enzyme-producing organisms. For example, to reduce operation cost in microbial cellulase production, the solid-state fermentation (SSF) decreases operation cost to one-tenth of submerged fermentation. SSF can also play an instrumental role as natural baseline for growing cellulase producers in a bioreactor in order to improvement of production (Viniegra-González et al. 2003).
The methodology for the design of enzyme recovery and purification is a new area in biotechnology research, because its industrial production is expensive and the waste raw materials are enriched with enzymes (Klein-Marcuschamer et al. 2012, Singhania et al. 2010; Garcia-Galan et al. 2011). Thus, the concepts of immobilization, purification and enzyme recovery as a general principle in bioactive materials production are very important and need high attention (Couto and Sanromán 2005; Sharma et al. 2015). Recently, Saengsanga et al. characterized alkaline-lipase product by engineered E. coli and showed that the recombinant lipase has the highest enzyme activity than the wild-type E1PA lipase from lipid-rich food wastes such as oil cakes. Moreover, several factors have been characterized such as optimal pH, optimal temperature, and the presence of heavy metals in order to maximize lipase activity (Saengsanga et al. 2016).
Twenty percent of the annual fruit and vegetable waste are generated in the USA, Australia, Canada and New Zealand reported to be due to the production and harvesting, and 52% of it as the total loss of production (Panda et al. 2016).
Agriculture wastes are rich in organic components including carbohydrate, proteins and fats, and antioxidants as valuable bioactive materials (Wijngaard et al. 2009). High-value products may be categorized into enzymes, organic acids, biofuel and bioelectricities. One of the most important products of agriculture waste is enzymes such as amylase, lignocelluloses, pectinase, tannase, invertase, protease and lipase (Howard et al. 2004; Milala et al. 2005; Oliveira et al. 2006; Nandini and Nandini 2015).
Recently, oligo- and polysaccharides such as starch were hydrolyzed into low molecular weight sugars including glucose, maltose and fructose by amylolytic enzymes. Erdal et al. proposed the use of Penicillium expansum in SSF using Eriobotrya japonica kernels for the production of α-amylase (Erdal and Taskin 2010). In the recent study, to increase α-amylase production, α-amylase genes of B. subtilis was cloned in E. coli HB101 which can be used in waste biofermenter for α-amylase production (Rabbani et al. 2011). Among agriculture wastes, karat processing waste, lemon peel, and coffee husk are commonly utilized for lipase production (Kumar and Kesavapillai 2012; Panda et al. 2016; Salihu et al. 2012). E. coli has been introduced as a relevant host in the cloning and expression of lipase gene of Bacillus stearothermophilus L1 (Jarosch et al. 2000). It has showed high specific gravity to purified enzyme (1700 units/mg protein) and calcium-dependent thermostability when olive oil was used as a substrate (Kim et al. 2000).
Livestock waste is used as an organic component for achieving valuable biofuel and combustible biogas by biochemical and thermochemical platforms (Cantrell et al. 2008). Methane (CH4) and carbon dioxide (CO2), two major members of GHGs, uses in Anaerobic digestion (AN) for biofuel production in three steps: hydrolysis, fermentation and methanogenesis (Kamali et al. 2016). In hydrolysis, livestock organic components are converted into soluble components. The second step involves that the acidogenic and acetogenic bacteria synthesize VFAs, organic acids and alcohol. Finally, H4 (60–70%) and CO2 (30–40%) and other by-products are produced by methanogens, which requires some essential factors like pH, temperature, organic loading rate, hydraulic retention time and change in influent materials (Cantrell et al. 2008; Cho et al. 2005).
Production of biohydrogen and bioelectricity from municipal waste
Hydrogen is an environmentally friendly gas (Kapdan and Kargi 2006). Nowadays, the mixed methane/hydrogen, as a renewable energy resource, is a potential substitute for biogas and petrochemical-based fuels in automotive engines (Caputo et al. 2016). As shown in Fig. 3B, hydrogen can be produced from water, sludge and recently, agriculture/food industry waste, on several platforms such as chemical, thermochemical and biochemical (Kapdan and Kargi 2006; Funk 2001; Cai et al. 2004). Biochemical routes, known as biological methods, have advantages for hydrogen production including specific conversion and use of mild condition, compared to chemical methods with high temperature (Abanades and Flamant 2006). The bioproduction strategies are the center of attention, since MSW and sewage sludge have been introduced as the suitable feedstock for biofuel production such as biohydrogen. Biohydrogen products can be achieved by following three different routes: (i) dark fermentation, (ii) biophotolysis, (iii) photo fermentation, while the certain routes influenced by together are shown in Fig. 3B (Levin et al. 2004).
While pure raw materials are expensive for biohydrogen production, the waste materials are cost-effective. Thus, they can serve as new metabolite for renewable resources with potential of industrial application. A summary of major waste materials and production routes for biohydrogen production as biofuel is illustrated in Fig. 4. Due to the use of inexpensive waste raw materials, application of these conversional routes for bio-based products production, from any waste, indicates that the technology for this process needs further attention and developments (Kapdan and Kargi 2006).
According to Washington et al., (Logroño et al. 2015) microbial fuel cells (MFC) used as an electrobiochemical device can generate bioelectricity from vegetable and fruit waste using high Adean soil. In the MFC, electrochemically active microorganisms (EAM) are used to generate electricity. In addition, they have application in bioelectrocatalysis and bioremediation, and MFCs can be used to obtain biohydrogen (Doyle and Marsili 2015; Logroño et al. 2015; Call et al. 2009). The EAMs can be arose in the wastewater and seawater sediments, an anaerobic condition, to eliminate O2. The presence of aerobic microorganisms is required to create a sufficient barrier to O2 diffusion to maintain anaerobic conditions (Doyle and Marsili 2015). The replacement of EAMs in wastewater and improvement of optimum growth rate to achieve bioelectricity generation is very applicable for waste treatment (Logroño et al. 2015).
Conclusion
Since the second generation, development of biotechnological methods has successfully converted various waste components, as raw materials, into valuable biomaterials, providing suitable feedstocks instead of petroleum. While biorefinery studies indicate that thermochemical platforms are very applicable, the increase in air pollution and global warming are two major obstacles to scale up biofuel production. However, this requires development of new approaches which reduce or remove the generation of GHGs and further attention should be to bioconversion of MSW as raw material for biofuel production in which carried out by microorganisms.
In food organic components, industrial and agriculture waste are abundant and can be separately isolated using various efficient methods for final production. Recently, the process design and parameter optimization for the development of biorefinery processes described by advanced biotechnology microalgae-based biorefinery methods have been developed. It has been suggested also that the production of microalgae biofuels is economically feasible, while several problems exists for utilization of microalgae-based biorefinery technologies. Although utilization of microalgae for biohydrogen products has two disadvantages, the first being low hydrogen production potential and the other, no waste utilization, biohydrogen production by microalgae is considered as a profitable and economical method. This is because microalgae can be used in water as a renewable resources and CO2 being utilized as one of the major air pollution elements.
Energy production is challenging for urban and industrial use. Biotechnology processes for bioenergy production have been largely employed to overcome the demand of biodiesel, through biomass conversion as a new energy source. Thus, biotechnology-based processes have been the best source for replacing thermos and chemical processes in biorefinery, focusing on several challenges, which arises in the second-generation biorefinery. Pretreatment processes and bioengineered enzymes will enhance the efficiency and reduce costs of bulk production.
References
Abanades S, Flamant G (2006) Thermochemical hydrogen production from a two-step solar-driven water-splitting cycle based on cerium oxides. Sol Energy 80:1611–1623
Alper H, Stephanopoulos G (2009) Engineering for biofuels: exploiting innate microbial capacity or importing biosynthetic potential? Nat Rev Microbiol 7:715–723
Annamalai N, Rajeswari MV, Elayaraja S, Balasubramanian T (2013) Thermostable, haloalkaline cellulase from Bacillus halodurans CAS 1 by conversion of lignocellulosic wastes. Carbohydr Polym 94:409–415
Antoni D, Zverlov VV, Schwarz WH (2007) Biofuels from microbes. Appl Microbiol Biotechnol 77:23–35
Arad SM, Levy-Ontman O (2010) Red microalgal cell-wall polysaccharides: biotechnological aspects. Curr Opin Biotechnol 21:358–364
Babaki M, Yousefi M, Habibi Z, Mohammadi M (2017) Process optimization for biodiesel production from waste cooking oil using multi-enzyme systems through response surface methodology. Renew Energy 105:465–472
Busch T, Cengel KA, Finlay J (2009) Pheophorbide a as a photosensitizer in photodynamic therapy: in vivo considerations. Cancer Biol Ther 8:540–542
Cai M, Liu J, Wei Y (2004) Enhanced biohydrogen production from sewage sludge with alkaline pretreatment. Environ Sci Technol 38:3195–3202
Call DF, Wagner RC, Logan BE (2009) Hydrogen production by geobacter species and a mixed consortium in a microbial electrolysis cell. Appl Environ Microbiol 75:7579–7587
Cantrell KB, Ducey T, Ro KS, Hunt PG (2008) Livestock waste-to-bioenergy generation opportunities. Biores Technol 99:7941–7953
Caputo G, Mazzei D, Sgroi MF (2016) Methane/hydrogen mixtures from concentrated solar energy: the METISOL project. Enriched methane. Springer, Berlin
Chen C-Y, Yeh K-L, Aisyah R, Lee D-J, Chang J-S (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Biores Technol 102:71–81
Cho Y, Young J, Jordan J, Moon H (2005) Factors affecting measurement of specific methanogenic activity. Water Sci Technol 52:435–440
Chochois V, Dauvillee D, Beyly A, Tolleter D, Cuine S, Timpano H, Ball S, Cournac L, Peltier G (2009) Hydrogen production in Chlamydomonas: photosystem II-dependent and-independent pathways differ in their requirement for starch metabolism. Plant Physiol 151:631–640
Christenson L, Sims R (2011) Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol Adv 29:686–702
Chylenski P, Forsberg Z, Ståhlberg J, Várnai A, Lersch M, Bengtsson O, Sæbø S, Horn SJ, Eijsink VG (2017) Development of minimal enzyme cocktails for hydrolysis of sulfite-pulped lignocellulosic biomass. J Biotechnol 246:16–23
Cirino PC, Chin JW, Ingram LO (2006) Engineering Escherichia coli for xylitol production from glucose-xylose mixtures. Biotechnol Bioeng 95:1167–1176
Conesa JA, Rey L (2015) Thermogravimetric and kinetic analysis of the decomposition of solid recovered fuel from municipal solid waste. J Therm Anal Calorim 120:1233–1240
Couto SR, Sanromán MA (2005) Application of solid-state fermentation to ligninolytic enzyme production. Biochem Eng J 22:211–219
Doyle LE, Marsili E (2015) Methods for enrichment of novel electrochemically-active microorganisms. Biores Technol 195:273–282
Dunahay TG, Jarvis EE, Roessler PG (1995) Genetic transformation of the diatoms Cyclotella cryptica and Navicula saprophila. J Phycol 31:1004–1012
Dutta K, Daverey A, Lin J-G (2014) Evolution retrospective for alternative fuels: first to fourth generation. Renew Energy 69:114–122
Erdal S, Taskin M (2010) Production of alpha-amylase by Penicillium expansum MT-1 in solid-state fermentation using waste Loquat (Eriobotrya japonica Lindley) kernels as substrate. Romanian Biotechnol Lett 15:5342–5350
Fan X, Niehus X, Sandoval G (2012) Lipases as biocatalyst for biodiesel production. Methods Mol Biol 861:471–483
Fulda M, Schnurr J, Abbadi A, Heinz E (2004) Peroxisomal Acyl-CoA synthetase activity is essential for seedling development in Arabidopsis thaliana. Plant Cell 16:394–405
Funk JE (2001) Thermochemical hydrogen production: past and present. Int J Hydrogen Energy 26:185–190
Gallezot P (2012) Conversion of biomass to selected chemical products. Chem Soc Rev 41:1538–1558
Gan S-Y, Lim P-E, Phang S-M (2016) Genetic and metabolic engineering of microalgae. Algae biotechnology. Springer, Berlin
Garcia-Galan C, Berenguer-Murcia Á, Fernandez-Lafuente R, Rodrigues RC (2011) Potential of different enzyme immobilization strategies to improve enzyme performance. Adv Synth Catal 353:2885–2904
Germain V, Rylott EL, Larson TR, Sherson SM, Bechtold N, Carde JP, Bryce JH, Graham IA, Smith SM (2001) Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid β-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J 28:1–12
Goo BG, Baek G, Choi DJ, Park YI, Synytsya A, Bleha R, Seong DH, Lee C-G, Park JK (2013) Characterization of a renewable extracellular polysaccharide from defatted microalgae Dunaliella tertiolecta. Biores Technol 129:343–350
Gudynaite-Savitch L, White TC (2016) Fungal biotechnology for industrial enzyme production: focus on (Hemi) cellulase production strategies, advances and challenges. Gene expression systems in fungi: advancements and applications. Springer, Berlin
Guo W, Sheng J, Zhao H, Feng X (2016) Metabolic engineering of Saccharomyces cerevisiae to produce 1-hexadecanol from xylose. Microb Cell Fact 15:1
Haro P, Ollero P, Villanueva Perales ÁL, Vidal-Barrero F (2013) Potential routes for thermochemical biorefineries. Biofuels Bioprod Biorefin 7:551–572
Hetzler S, Steinbuchel A (2013) Establishment of cellobiose utilization for lipid production in Rhodococcus opacus PD630. Appl Environ Microbiol 79:3122–3125
Ho S-H, Chen C-Y, Chang J-S (2012) Effect of light intensity and nitrogen starvation on CO 2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Biores Technol 113:244–252
Holder JW, Ulrich JC, Debono AC, Godfrey PA, Desjardins CA, Zucker J, Zeng Q, Leach AL, Ghiviriga I, Dancel C (2011) Comparative and functional genomics of Rhodococcus opacus PD630 for biofuels development. PLoS Genet 7:e1002219
Hong C-O, Nam M-H, Oh J-S, Lee J-W, Kim C-T, Park K-W, Lee D-H, Lee K-W (2016) Pheophorbide a from Capsosiphon fulvescens inhibits advanced glycation end products mediated endothelial dysfunction. Planta Med 82:46–57
Howard R, Abotsi E, van Rensburg EJ, Howard S (2003) Lignocellulose biotechnology: issues of bioconversion and enzyme production. Afr J Biotechnol 2:602–619
Howard R, Abotsi E, van Rensburg EJ, Howard S (2004) Lignocellulose biotechnology: issues of bioconversion and enzyme production. Afr J Biotechnol 2:602–619
Hung C-H, Kanehara K, Nakamura Y (2016) In vivo reconstitution of algal triacylglycerol production in Saccharomyces cerevisiae. Front Microbiol 7:1–9
Ivanov V, Stabnikov V, Ahmed Z, Dobrenko S, Saliuk A (2015) Production and applications of crude polyhydroxyalkanoate-containing bioplastic from the organic fraction of municipal solid waste. Int J Environ Sci Technol 12:725–738
Jang Y-S, Park JM, Choi S, Choi YJ, Cho JH, Lee SY (2012) Engineering of microorganisms for the production of biofuels and perspectives based on systems metabolic engineering approaches. Biotechnol Adv 30:989–1000
Jarosch M, Egelseer EM, Mattanovich D, Sleytr UB, SáRA M (2000) S-layer gene sbsC of Bacillus stearothermophilus ATCC 12980: molecular characterization and heterologous expression in Escherichia coli. Microbiology 146:273–281
Jin M, Slininger PJ, Dien BS, Waghmode S, Moser BR, Orjuela A, Da Costa Sousa L, Balan V (2015) Microbial lipid-based lignocellulosic biorefinery: feasibility and challenges. Trends Biotechnol 33:43–54
John RP, Anisha G, Nampoothiri KM, Pandey A (2011) Micro and macroalgal biomass: a renewable source for bioethanol. Biores Technol 102:186–193
Kamali M, Gameiro T, Costa MEV, Capela I (2016) Anaerobic digestion of pulp and paper mill wastes—an overview of the developments and improvement opportunities. Chem Eng J 298:162–182
Kaparaju P, Serrano M, Thomsen AB, Kongjan P, Angelidaki I (2009) Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Biores Technol 100:2562–2568
Kapdan IK, Kargi F (2006) Bio-hydrogen production from waste materials. Enzyme Microb Technol 38:569–582
Karbassi A, Nasrabadi T, Rezai M, Modabberi S (2014) Pollution with metals (As, Sb, Hg, Zn) in agricultural soil located close to Zarshuran gold mine, Iran. Environ Eng Manag J (EEMJ) 13:115–122
Kim M-H, Kim H-K, Lee J-K, Park S-Y, Oh T-K (2000) Thermostable lipase of Bacillus stearothermophilus: high-level production, purification, and calcium-dependent thermostability. Biosci Biotechnol Biochem 64:280–286
Klein-Marcuschamer D, Oleskowicz-Popiel P, Simmons BA, Blanch HW (2012) The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol Bioeng 109:1083–1087
Kobayashi K, Hattori T, Honda Y, Kirimura K (2014) Oxalic acid production by citric acid-producing Aspergillus niger overexpressing the oxaloacetate hydrolase gene oahA. J Ind Microbiol Biotechnol 41:749–756
Kubicek CP, Schreferl-Kunar G, Wohrer W, Rohr M (1988) Evidence for a cytoplasmic pathway of oxalate biosynthesis in Aspergillus niger. Appl Environ Microbiol 54:633–637
Kumar R, Kesavapillai B (2012) Stimulation of extracellular invertase production from spent yeast when sugarcane pressmud used as substrate through solid state fermentation. SpringerPlus 1:1–6
Kunjapur AM, Eldridge RB (2010) Photobioreactor design for commercial biofuel production from microalgae. Ind Eng Chem Res 49:3516–3526
Kurosawa K, Wewetzer SJ, Sinskey AJ (2013) Engineering xylose metabolism in triacylglycerol-producing Rhodococcus opacus for lignocellulosic fuel production. Biotechnol Biofuels 6:1
Lee M, Lee D, Cho J, Kim S, Park C (2013) Enzymatic biodiesel synthesis in semi-pilot continuous process in near-critical carbon dioxide. Appl Biochem Biotechnol 171:1118–1127
Lei A, Chen H, Shen G, Hu Z, Chen L, Wang J (2012) Expression of fatty acid synthesis genes and fatty acid accumulation in Haematococcus pluvialis under different stressors. Biotechnol Biofuels 5:1
Leung CCJ, Cheung ASY, Zhang AY-Z, Lam KF, Lin CSK (2012) Utilisation of waste bread for fermentative succinic acid production. Biochem Eng J 65:10–15
Levin DB, Pitt L, Love M (2004) Biohydrogen production: prospects and limitations to practical application. Int J Hydrogen Energy 29:173–185
Logroño W, Ramírez G, Recalde C, Echeverría M, Cunachi A (2015) Bioelectricity generation from vegetables and fruits wastes by using single chamber microbial fuel cells with high Andean soils. Energy Procedia 75:2009–2014
Lotfy WA, Ghanem KM, El-Helow ER (2007) Citric acid production by a novel Aspergillus niger isolate: II. Optimization of process parameters through statistical experimental designs. Biores Technol 98:3470–3477
Milala M, Shugaba A, Gidado A, Ene A, Wafar J (2005) Studies on the use of agricultural wastes for cellulase enzyme production by Aspergillus niger. Res J Agric Biol Sci 1:325–328
Moazami N, Ashori A, Ranjbar R, Tangestani M, Eghtesadi R, Nejad AS (2012) Large-scale biodiesel production using microalgae biomass of Nannochloropsis. Biomass Bioenergy 39:449–453
More T, Yan S, Tyagi R, Surampalli R (2016) Biopolymer production kinetics of mixed culture using wastewater sludge as a raw material and the effect of different cations on biopolymer applications in water and wastewater treatment. Water Environ Res 88:425–437
Nandini S, Nandini K (2015) Food and agriculture residue (FAR): a potential substrate for tannase and gallic acid production using competent microbes. J Bioprocess Biotech 5:1–8
Narra M, Dixit G, Divecha J, Madamwar D, Shah AR (2012) Production of cellulases by solid state fermentation with Aspergillus terreus and enzymatic hydrolysis of mild alkali-treated rice straw. Biores Technol 121:355–361
Oliveira LA, Porto AL, Tambourgi EB (2006) Production of xylanase and protease by Penicillium janthinellum CRC 87M-115 from different agricultural wastes. Biores Technol 97:862–867
Palmer JD, Brigham CJ (2016) Feasibility of triacylglycerol production for biodiesel, utilizing Rhodococcus opacus as a biocatalyst and fishery waste as feedstock. Renew Sustain Energy Rev 56:922–928
Panda SK, Mishra SS, Kayitesi E, Ray RC (2016) Microbial-processing of fruit and vegetable wastes for production of vital enzymes and organic acids: biotechnology and scopes. Environ Res 146:161–172
Pedersen H, Christensen B, Hjort C, Nielsen J (2000) Construction and characterization of an oxalic acid nonproducing strain of Aspergillus niger. Metab Eng 2:34–41
Pimentel D, Patzek TW (2005) Ethanol production using corn, switchgrass, and wood; biodiesel production using soybean and sunflower. Nat Resour Res 14:65–76
Poggi-Varaldo HM, Munoz-Paez KM, Escamilla-Alvarado C, Robledo-Narváez PN, Ponce-Noyola MT, Calva-Calva G et al (2014) Biohydrogen, biomethane and bioelectricity as crucial components of biorefinery of organic wastes: a review. Waste Manag Res 32:353–365
Quinn JM, Merchant S (1995) Two copper-responsive elements associated with the Chlamydomonas Cyc6 gene function as targets for transcriptional activators. The Plant Cell 7:623–628
Ra CH, Kang C-H, Jung J-H, Jeong G-T, Kim S-K (2016) Enhanced biomass production and lipid accumulation of Picochlorum atomus using light-emitting diodes (LEDs). Bioresour Technol 218:1279–1283
Rabbani M, Mirmohammad Sadeghi H, Moazen F, Rahimi M, Salehi G (2011) Cloning and expression of randomly mutated bacillus subtilis-amylase genes in HB101. Biotechnol Res Int 2011:1–5
Radakovits R, Jinkerson RE, Darzins A, Posewitz MC (2010) Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 9:486–501
Ravindran R, Jaiswal AK (2016) Exploitation of food industry waste for high-value products. Trends Biotechnol 34:58–69
Renuka N, Sood A, Prasanna R, Ahluwalia A (2015) Phycoremediation of wastewaters: a synergistic approach using microalgae for bioremediation and biomass generation. Int J Environ Sci Technol 12:1443–1460
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632
Robins M, Rickus J (2015) Development of a Novel Enzymatic Pre-treatment For Lignocellulosic Biomass. The Summer Undergraduate Research Fellowship (SURF) Symposium. Paper 20. http://docs.lib.purdue.edu/surf/2015/presentations/20. Accessed 15 Sept 2015
Saengsanga T, Siripornadulsil W, Siripornadulsil S (2016) Molecular and enzymatic characterization of alkaline lipase from Bacillus amyloliquefaciens E1PA isolated from lipid-rich food waste. Enzyme Microb Technol 82:23–33
Salihu A, Alam MZ, Abdulkarim MI, Salleh HM (2012) Lipase production: an insight in the utilization of renewable agricultural residues. Resour Conserv Recycl 58:36–44
Sambusiti C, Monlau F, Barakat A (2016) Bioethanol fermentation as alternative valorization route of agricultural digestate according to a biorefinery approach. Biores Technol 212:289–295
Sanders J, Scott E, Weusthuis R, Mooibroek H (2007) Bio-refinery as the bio-inspired process to bulk chemicals. Macromol Biosci 7:105–117
Sarkar D, Shimizu K (2015) An overview on biofuel and biochemical production by photosynthetic microorganisms with understanding of the metabolism and by metabolic engineering together with efficient cultivation and downstream processing. Bioresour Bioprocess 2:1–19
Schmid RD, Urlacher V (2007) Modern biooxidation: enzymes, reactions and applications. Wiley, Hoboken
Seong PJ, Jeon BW, Lee M, Cho DH, Kim DK, Jung KS, Kim SW, Han SO, Kim YH, Park C (2011) Enzymatic coproduction of biodiesel and glycerol carbonate from soybean oil and dimethyl carbonate. Enzyme Microb Technol 48:505–509
Sharma R, Rawat R, Bhogal RS, Oberoi HS (2015) Multi-component thermostable cellulolytic enzyme production by Aspergillus niger HN-1 using pea pod waste: appraisal of hydrolytic potential with lignocellulosic biomass. Process Biochem 50:696–704
Singhania RR, Sukumaran RK, Patel AK, Larroche C, Pandey A (2010) Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme Microb Technol 46:541–549
Singhania RR, Patel AK, Christophe G, Fontanille P, Larroche C (2013) Biological upgrading of volatile fatty acids, key intermediates for the valorization of biowaste through dark anaerobic fermentation. Biores Technol 145:166–174
Slack R, Gronow J, Voulvoulis N (2005) Household hazardous waste in municipal landfills: contaminants in leachate. Sci Total Environ 337:119–137
Slininger P, Bolen P, Kurtzman C (1987) Pachysolen tannophilus: properties and process considerations for ethanol production from d-xylose. Enzyme Microb Technol 9:5–15
Smith AM, Zeeman SC, Smith SM (2005) Starch degradation. Annu Rev Plant Biol 56:73–98
Sonderegger M, Jeppsson M, Larsson C, Gorwa-Grauslund MF, Boles E, Olsson L, Spencer-Martins I, Hahn-Hägerdal B, Sauer U (2004) Fermentation performance of engineered and evolved xylose-fermenting Saccharomyces cerevisiae strains. Biotechnol Bioeng 87:90–98
Souza CP, Almeida BC, Colwell RR, Rivera IN (2011) The importance of chitin in the marine environment. Mar Biotechnol 13:823–830
Stuart PR, El-Halwagi MM (2012) Integrated biorefineries: design, analysis, and optimization. CRC Press, Boca Raton
Stuart BJ, Kosson DS (1994) Characterization of municipal waste combustion air pollution control residues as a function of particle size. Combust Sci Technol 101:527–548
Tabatabaei M, Tohidfar M, Jouzani GS, Safarnejad M, Pazouki M (2011) Biodiesel production from genetically engineered microalgae: future of bioenergy in Iran. Renew Sustain Energy Rev 15:1918–1927
Tannin-Spitz T, Bergman M, Van-Moppes D, Grossman S, Arad SM (2005) Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J Appl Phycol 17:215–222
Tsuruta H, Paddon CJ, Eng D, Lenihan JR, Horning T, Anthony LC, Regentin R, Keasling JD, Renninger NS, Newman JD (2009) High-level production of amorpha-4, 11-diene, a precursor of the antimalarial agent artemisinin, in Escherichia coli. PLoS ONE 4:e4489
Viniegra-González G, Favela-Torres E, Aguilar CN, De Jesus Rómero-Gomez S, Dıaz-Godınez G, Augur C (2003) Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem Eng J 13:157–167
Walfridsson M, Bao X, Anderlund M, Lilius G, Bulow L, Hahn-Hägerdal B (1996) Ethanolic fermentation of xylose with Saccharomyces cerevisiae harboring the Thermus thermophilus xylA gene, which expresses an active xylose (glucose) isomerase. Appl Environ Microbiol 62:4648–4651
Wang ZT, Ullrich N, Joo S, Waffenschmidt S, Goodenough U (2009) Algal lipid bodies: stress induction, purification, and biochemical characterization in wild-type and starchless Chlamydomonas reinhardtii. Eukaryot Cell 8:1856–1868
Westfall PJ, Pitera DJ, Lenihan JR, Eng D, Woolard FX, Regentin R, Horning T, Tsuruta H, Melis DJ, Owens A (2012) Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc Natl Acad Sci 109:E111–E118
Whitney SM, Houtz RL, Alonso H (2011) Advancing our understanding and capacity to engineer nature’s CO2-sequestering enzyme, Rubisco. Plant Physiol 155:27–35
Wijngaard HH, RößLE C, Brunton N (2009) A survey of Irish fruit and vegetable waste and by-products as a source of polyphenolic antioxidants. Food Chem 116:202–207
Williams RB (2007) Biofuels from municipal wastes. Background discussion paper. University of California at Davis and California Biomass Initiative
Willke T, Vorlop K-D (2004) Industrial bioconversion of renewable resources as an alternative to conventional chemistry. Appl Microbiol Biotechnol 66:131–142
Xue J, Niu Y-F, Huang T, Yang W-D, Liu J-S, Li H-Y (2015) Genetic improvement of the microalga Phaeodactylum tricornutum for boosting neutral lipid accumulation. Metab Eng 27:1–9
Yan Q, Yang R, Zhang Y, Umar A, Huang Z, Wang Q (2016) A comprehensive review on selective catalytic reduction catalysts for NOx emission abatement from municipal solid waste incinerators. Environ Prog Sustain Energy 35:1061–1069
Yang X, Lee SJ, Yoo HY, Choi HS, Park C, Kim SW (2014) Biorefinery of instant noodle waste to biofuels. Bioresour Technol 159:17–23
Yang J, Nie Q, Liu H, Xian M, Liu H (2016a) A novel MVA-mediated pathway for isoprene production in engineered E. coli. BMC Biotechnol 16:1
Yang J, Pan Y, Bowler C, Zhang L, Hu H (2016b) Knockdown of phosphoenolpyruvate carboxykinase increases carbon flux to lipid synthesis in Phaeodactylum tricornutum. Algal Res 15:50–58
Yen H-W, Hu I-C, Chen C-Y, Ho S-H, Lee D-J, Chang J-S (2013) Microalgae-based biorefinery–from biofuels to natural products. Biores Technol 135:166–174
Yuan L, Voelker TA, Hawkins DJ (1995) Modification of the substrate specificity of an acyl-acyl carrier protein thioesterase by protein engineering. Proc Natl Acad Sci 92:10639–10643
Zhang M, Eddy C, Deanda K, Finkelstein M, Picataggio S (1995) Metabolic engineering of a pentose metabolism pathway in ethanologenic Zymomonas mobilis. Science 267:240
Zhang G, French WT, Hernandez RE, Hall J, Sparks D, Holmes WE (2011) Microbial lipid production as biodiesel feedstock from N-acetylglucosamine by oleaginous microorganisms. J Chem Technol Biotechnol 86:642–650
Zhang J, Li K, Huang J, Wang D (2015) Optimization of fermentation medium for citric acid production by Aspergillus niger. Advances in applied biotechnology. Springer, Berlin
Zou H, Zhao G, Liu H, Xian M (2016) Bulk chemical production: chemo-and bio-integrated strategies. Sustainable production of bulk chemicals. Springer, Berlin
Acknowledgements
This work was financially supported by the Tehran Urban Research & Planning Center, Tehran, Iran and supported by the Tehran University of Medical Science, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Xu Han.
Rights and permissions
About this article
Cite this article
Haddadi, M.H., Aiyelabegan, H.T. & Negahdari, B. Advanced biotechnology in biorefinery: a new insight into municipal waste management to the production of high-value products. Int. J. Environ. Sci. Technol. 15, 675–686 (2018). https://doi.org/10.1007/s13762-017-1424-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1424-x