Abstract
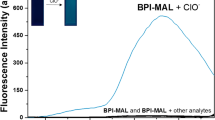
A new fluorescent (TMCD) probe has been developed for the detection of hypochlorous acid (HClO) with sensitive response toward HClO. The probe color changes from green to yellow during the addition of HClO following PET OFF mechanism. The chemodosimeter promoted a selective response to hypochlorous acid compared to other ROS and RNS with a detection limit at 89 nM. The binding mechanism of probe with guest molecules has been confirmed by NMR spectroscopy, Mass spectroscopy and DFT calculations.
Graphical abstract

Research highlights
-
1.
The sensor TMDC was synthesized by a simple method with high yield.
-
2.
The sensor colorimetric and fluorimetrically detection of HClO very easily in aqueous medium.
-
3.
The probe was easily recognized toxic ROS form of HClO compare than other ROS.
-
4.
The HClO detection of limit 89nM in aqueous medium was reported.









Similar content being viewed by others
References
Y.K. Yue, F.J. Huo, C.X. Yin, J.O. Escobedo, R.M. Strongin, Recent progress in chromogenic and fluorogenicchemosensors for hypochlorous acid. Analyst 141, 1859–1873 (2019). https://doi.org/10.1039/C6AN00158K
N.J. Mehta, K. Asmaro, D.J. Hermiz, M.M. Njus, A.H. Saleh, K.H. Beningo, D. Njus, Hypochlorite covertedcysteinyl-dopamine into cytotoxic product a possible factor in parkinson disease free radic. Biol. Med. 101, 44–52 (2016). https://doi.org/10.1016/j.freeradbiomed.2016.09.023
M.J. Steinbeck, L.J. Nesti, P.F. Sharkey, J. Parvizi, Myeloperoxidase and chlorinated peptides in osteoarthritis: potential biomarkers of the disease. J. Orthop. Res. 25, 1128–1135 (2007). https://doi.org/10.1002/jor.20400
W.H.F. Sutherland, S.A. Jong, R.J. Walker, Hypochlorous acid and 3,4-dihydroxyphenylalanine increase the formation of serum protein lipofuscin-like fluorophores in vitro. Ren. Fail. 27, 239–246 (2005). https://doi.org/10.1081/JDI-49544
Y.W. Yap, M. Whiteman, B.H. Bay, Y. Li, F.S. Sheu, R.Z. Qi, C.H. Tan, N.S. Cheung, Hypochlorous acid induces apoptosis of cultured cortical neurons through activation of calpains and rupture of lysosomes. J. Neurochem. 98, 1597–1609 (2006). https://doi.org/10.1111/j.1471-4159.2006.03996.x
C.A. Hitchon, H.S.E. Gabalawy, Oxidation in rheumatoid arthritis. Arthritis. Res. Ther. 6, 265–278 (2004). https://doi.org/10.1186/ar1447
J. Zhou, Q. Wang, Y. Ding, M.H. Zou, Hypochlorous acid via peroxynitrite activates protein kinase Cθ and insulin resistance in adipocytes. J. Mol. Endocrinol. 54, 25–37 (2015). https://doi.org/10.1530/JME-14-0213
C.H. Sam, H.K. Lu, The role of hypochlorous acid as one of the reactive oxygen species in periodontal disease. J. Dent. Sci. 4, 45–54 (2009). https://doi.org/10.1016/S1991-7902(09)60008-8
S. Hammerschmidt, N. Buchler, H. Wahn, Tissue lipid peroxidation and reduced glutathione depletion in hypochlorite-induced lung injury. Chest 121, 573–581 (2002). https://doi.org/10.1378/chest.121.2.573
Y. Lou, C. Wang, S. Chi, S. Li, Z. Mao, Z. Liu, Construction of a two-photon fluorescent probe for ratiometric imaging of hypochlorous acid in alcohol-induced liver injury. Chem. Commun. 55, 12912–12915 (2019). https://doi.org/10.1039/C9CC06888K
A. Ulfig, L.I. Leichert, The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens. Cell. Mol. Life Sci. 13, 1–30 (2020). https://doi.org/10.1007/s00018-020-03591-y
M.S. Block, B.G. Rowan, Hypochlorous acids review. J. Oral Maxillofac. Surg. 78, 1461–1466 (2020). https://doi.org/10.1016/j.joms.2020.06.029
Y. Hu, G. Xie, D.M. Stanbury, Oxidations at sulfur centers by aqueous hypochlorous acid and hypochlorite: Cl+ versus O atom transfer. Inorg. Chem. 56, 4047–4056 (2017). https://doi.org/10.1021/acs.inorgchem.6b03182
C.L. Hawkins, M.J. Davies, Hypochlorite-induced damage to DNA, RNA, and polynucleotides formation of chloramines and nitrogen-centered radicals. Chem. Res. Toxicol. 15, 83–92 (2002). https://doi.org/10.1021/tx015548d
O. Ordeig, R. Mas, J. Gonzalo, Continuous detection of hypochlorous acid/hypochlorite for water quality monitoring and control. Electroanalysis 17, 1641–1648 (2005). https://doi.org/10.1002/elan.200403194
P. Soldatkin, D.V. Gorchkov, C. Martele, New enzyme potentiometric sensor for hypochlorite species detection sens. Actuators B 43, 99 (1997). https://doi.org/10.1016/S0925-4005(97)00144-5
N.O. Soto, B. Horstkotte, J.G. March, P.L.L. Alba, L.L. Martinez, V.C. Martin, An environmental friendly method for the automatic determination of hypochlorite in commercial products using multisyringe flow injection analysis. Anal. Chim. Acta. 611, 182–186 (2008). https://doi.org/10.1016/j.aca.2008.01.073
J.B. Claver, M.C.V. Mirn, L.F.C. Vallvey, Determination of hypochlorite in water using a chemiluminescent test strip. Anal. Chim. Acta 522, 267–273 (2004). https://doi.org/10.1016/j.aca.2004.06.051
X. Chen, X. Tian, I. Shin, J. Yoon, Fluorescent and luminescent probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 40, 4783–4804 (2011). https://doi.org/10.1039/C1CS15037E
T. Watanabe, T. Idehara, Y. Yoshimura, H. Nakazawa, Simultaneous determination of chlorinedioxide and hypochlorite in water by high-performance liquid chromatography. J. Chromatogr. A 796, 397–400 (1998). https://doi.org/10.2478/s11532-006-0054-9
L. Nejdl, J. Sochor, O. Zitka, N. Cernei, B.R. Nedecky, P. Kopel, P. Babula, V. Adam, J. Hubalek, R. Kizek, Spectrometric and chromatographic study of reactive oxidants hypochlorousand and hypobromous acids and their interactions with taurine. Chromatographia 76, 363–373 (2013). https://doi.org/10.1007/s10337-012-2354-x
K. Tian, P.K. Dasgupta, Simultaneous flow-injection measurement of hydroxide, chloride, hypochlorite and chlorate in chlor–alkali cell effluents. Talanta 52, 623–630 (2000). https://doi.org/10.1016/S0039-9140(00)00399-4
X. Chen, T. Pradhan, F. Wang, J.S. Kim, J. Yoon, Fluorescent chemosensors on spiroring-opening of xanthenes and related derivatives. Chem. Rev. 112, 1910–1956 (2012). https://doi.org/10.1021/cr200201z
R. Zhang, B. Song, J. Yuan, Bioanalytical methods for hypochlorous acid detection: recent advances and challenges. Trends Analyt. Chem. 99, 1–33 (2018). https://doi.org/10.1016/j.trac.2017.11.015
M.F. Zhang, X. Liang, W. Zhang, Y.L. Wang, H. Wang, Y.H. Mohammed, B. Song, R. Zhang, J. Yuan, A unique iridium (III) complex-based chemosensor for multi-signal detection and multi-channel imaging of hypochlorous acid in liver injury. Biosens. Bioelectron. 87, 1005–1011 (2017). https://doi.org/10.1016/j.bios.2016.09.067
K. Ponnuvel, J. Ramamoorthy, G. Sivaraman, V. Padmini, Merocyanine dye-based fluorescent chemosensor for highly selective and sensitive detection of hypochlorous acid and imaging in live cells. ChemistrySelect 3(1), 91–95 (2018). https://doi.org/10.1002/slct.201701833
W.J. Zhang, C. Guo, L.B. Liu, J.G. Qin, C.L. Yang, Naked-eye visible and fluorometric dual-signaling chemodosimeter for hypochlorous acid based on water-soluble p-methoxyphenol derivative. Org. Biomol. Chem. 9, 5560–5563 (2011). https://doi.org/10.1039/C1OB05550J
U. Haldara, R. Sharmaa, B. Ruidasb, H. Lee, Toward rapid and selective detection of hypochlorous acid in pure aqueous media and its application to cell imaging: BODIPY-derived water-soluble macromolecular chemosensor with high sensitivity. Dyes Pigments 172, 107858 (2020). https://doi.org/10.1016/j.dyepig.2019.107858
H. Sun, H. Yu, H. Zhu, F. Ma, S. Zhang, D. Huang, S. Wang, Oxidative cleavage-based near-infrared fluorescent probe for hypochlorous acid detection and myeloperoxidase activity evaluation. Anal. Chem. 86, 671–677 (2014). https://doi.org/10.1021/ac403603r
Z. Zhang, Y. Zou, C. Deng, L. Meng, A simple rhodamine hydrazide-based turn-on fluorescent probe for HClO detection. Luminescence 31, 997–1004 (2016). https://doi.org/10.1002/bio.3064
J. Zhou, L. Li, W. Shi, X. Gao, X. Li, H. Ma, HClO can appear in the mitochondria of macrophages during bacterial infection as revealed by a sensitive mitochondrial-targeting fluorescent probe. Chem. Sci. 8, 4884–4888 (2015). https://doi.org/10.1039/C5SC01562F
Q. Xu, L. Kyung-Ah, L. Songyi, M.L. Kyung, L. Won-Jae, Y. Juyoung, A highly specific fluorescent probe for hypochlorous acid and its application in imaging microbe-induced HClO production. J. Am. Chem. Soc. 26, 9944–9949 (2013). https://doi.org/10.1021/ja404649m
Q. Xu, C.H. Heo, J.A. Kim, H.S. Lee, Y. Hu, D. Kim, M.K. Swamy, G. Kim, S.J. Nam, H.M. Kim, J. Yoon, A selective imidazoline-2-thione-bearing two-photon fluorescent probe for hypochlorous acid in mitochondria. Anal. Chem. 88, 6615–6620 (2016). https://doi.org/10.1002/bkcs.10926
W.C. Chen, V. Parthiban, W. Shu-Pao, A highly selective turn-on fluorescent probe for hypochlorous acid based on hypochlorous acid-induced oxidative intramolecular cyclization of boron dipyrromethene-hydrazone. Anal. Chim. Acta 882, 68–75 (2015). https://doi.org/10.1016/j.aca.2015.04.012
Y. Deng, F. Shumin, X. Qingfeng, G. Shengyi, F. Guoqiang, A novel reaction-based fluorescence probe for rapid imaging of HClO in live cells, animals, and injured liver tissues. Talanta 215, 120901 (2020). https://doi.org/10.1016/j.talanta.2020.12090
S.K. Yao, Q. Ying, A naphthalimide–rhodamine two-photon fluorescent turn-on probe for hypochlorous acid by desulfurization-cyclization and fluorescence resonance energy transfer. Sens. Actuators B Chem. 252, 877–885 (2017). https://doi.org/10.1039/c8an02196a
Y. Shiraishi, Y. Chiharu, H. Takayuki, A coumarin–dihydroperimidine dye as a fluorescent chemosensor for hypochlorite in 99% water. RSC Adv. 49, 28636–28641 (2019). https://doi.org/10.1039/c9ra05533a
H. Jia, X. Shuhe, F. Huan, M. Qingtao, D. Chengchen, Z. Zhiqiang, Z. Run, A fast response fluorescence probe specific for hypochlorous acid detection and its applications in bioimaging. Org. Biomol. Chem. 12, 2074–2082 (2018). https://doi.org/10.1002/asia.201500690
P. Wei, Y. Wei, X. Fengfeng, Z. Wei, L. Ruohan, Z. Datong, Y. Tao, Deformylation reaction-based probe for in vivo imaging of HClO. Chem. Sci. 2, 495–501 (2018). https://doi.org/10.1039/c7sc03784h
L. Shi, Y. Huijuan, Z. Xianqing, Y. Sheng, G. Shengzhao, X. Hua, Z. Kai, S. Guang, A novel ratiometric fluorescent probe based on thienocoumarin and its application for the selective detection of hypochlorite in real water samples and in vivo. New J. Chem. 16, 6232–6237 (2020). https://doi.org/10.1016/j.snb.2018.01.202
F. Zhang, X. Li, Z. Wenzhu, W. Yong-Lei, W. Haolu, H.M. Yousuf, S. Bo, Z. Run, Y. Jingli, A unique iridium (III) complex-based chemosensor for multi-signal detection and multi-channel imaging of hypochlorous acid in liver injury. Biosens. Bioelectron. 87, 1005–1011 (2017). https://doi.org/10.1016/j.bios.2016
X. Chen, W. Xiaochun, W. Shujuan, S. Wen, W. Ke, M. Huimin, A highly selective and sensitive fluorescence probe for the hypochlorite anion. Chem. Eur. J. 15, 4719–4724 (2008). https://doi.org/10.1002/chem.200701677
S. Bhaskar, S.S. Kumar, P.S. Kumar, K. Debaprasad, S. Nirmalendu, K. Snehadrinarayan, Highly selective detection of hypochlorous acid by a bis-heteroleptic Ru (II) complex of pyridyl-1, 2, 3-triazole ligand via C (sp2)–H hydroxylation. Inorg. Chem. 15, 9982–9991 (2019). https://doi.org/10.1021/acs.inorgchem.9b01125
A.P. De Silva, S. Thomas, G.D. Moody, Fluorescent PET (Photoinduced Electron Transfer) sensors as potent analytical tools. Analyst 12, 2385–2393 (2009). https://doi.org/10.1039/B912527M
S. Sarma, Y.J. Kim, M. Song, J.C. Ryu, Induction of apoptosis in human leukemia cells through the production of reactive oxygen species and activation of HMOX1 and Noxa by benzene, toluene, and o-xylene. Toxicology 280, 109–117 (2011). https://doi.org/10.1016/j.tox.2010.11.017
M.M. Ge, F. Hu, Z.Y. Lou, W. Xue, Xu.L. YuH, H.L. Wang, Role of Wnt/β-catenin signaling in the protective effect of epigallocatechin-3-gallate on lead-induced impairments of spine formation in the hippocampus of rats. RSC Adv. 5, 31622–31628 (2015). https://doi.org/10.1039/C5RA00315F
N. Eller, B. Netterstrøm, P. Laursen, Risk of chronic effects on the central nervous system at low toluene exposure. Occup. Med. 49, 389–395 (1999). https://doi.org/10.1093/occmed/49.6.389
B. Martin, M. Pearson, L. Kebejian, E. Golden, Nutrition and physiological function. Nat. Clin. Pract. Neurol. 3, 374–382 (2007). https://doi.org/10.1097/MCO.0b013e32831744ef
X. Huang, X. Gu, G. Zhang, D. Zhang, A highly selective fluorescence turn-on detection of cyanide based on the aggregation of tetraphenylethylene molecules induced by chemical reaction. Chem. Commun. 48, 12195–12197 (2012). https://doi.org/10.1039/C2CC37094H
G. Banuppriya, R. Sribalan, V. Padmini, V. Shanmugaiah, Biological evaluation and molecular docking studies of new curcuminoid derivatives: synthesis and characterization. Bioorg. Med. Chem. Lett. (2016). https://doi.org/10.1002/bio.3931
R.W. Boyle, I.R. Jonasson, The geochemistry of antimony and its use as an indicator element in geochemical prospecting. J. Geochem. Explor. 20, 223–302 (1984). https://doi.org/10.1016/0375-6742(84)90071-2
P. Perucci, E. Monaci, A. Onofri, C. Vischetti, C. Casucci, Changes in physico-chemical and biochemical parameters of soil following addition of wood ash: a field experiment. Eur. J. Agron. 28, 155–161 (2008). https://doi.org/10.1016/j.eja.2007.06.005
A.A. Melikian, K.G. Jordan, J. Braley, J. Rigotty, C.L. Meschter, S.S. Hecht, D. Hoffmann, Effects of catechol on the induction of tumors in mouse skin by 7, 8-dihydroxy-7, 8-dihydrobenzo [a] pyrenes. Carcinogenesis 10, 1897–1900 (1989). https://doi.org/10.1093/carcin/10.10.1897
X. Bao, X. Cao, Y. Yuan, B. Zhou, C. Huo, A water-soluble, highly sensitive and ultrafast fluorescence probe for imaging of mitochondrial hypochlorous acid. Sens. Actuators B Chem. (2021). https://doi.org/10.1016/j.snb.2021.130210
G. Tugcu, M.D. Ertürk, M.T. Saçan, On the aquatic toxicity of substituted phenols to chlorella vulgaris: QSTR with an extended novel data set and interspecies models. J. Hazard. Mater. 339, 122–130 (2017). https://doi.org/10.1016/j.jhazmat.2017.06.027
T. Lai, W. Cai, H. Du, J. Ye, Fe3O4 microspheres and graphene oxide encapsulated with chitosan: a new platform for sensitive determination of hydroquinone and catechol. Electroanalysis 26, 216–222 (2014). https://doi.org/10.1002/elan.201300444
V. Sethuraman, P. Muthuraja, M. Sethupathy, P. Manisankar, Development of biosensor for catechol using electrosynthesized poly (3-methylthiophene) and incorporation of LAC simultaneously. Electroanalysis 26, 1958–1965 (2014). https://doi.org/10.1002/elan.201400236
Y. Li, S. Feng, Y. Zhong, Y. Li, S. Li, Simultaneous and highly sensitive determination of hydroquinone and catechol using carboxyl functionalized graphene self-assembled monolayers. Electroanalysis 27, 2221–2229 (2015). https://doi.org/10.1002/elan.201500114
K.J. Rao, S. Paria, Aeglemarmelos leaf extract and plant surfactants mediated green synthesis of Au and Ag nanoparticles by optimizing process parameters using Taguchi method. ACS Sustain. Chem. Eng. 3, 483–491 (2015). https://doi.org/10.1021/acssuschemeng.5b00022
K. Bavarsad, G.E. Barreto, A. Sahebkar, Protective effects of curcumin against ischemia-reperfusion injury in the nervous system. Mol. Neurobiol. 56, 1391–1404 (2019). https://doi.org/10.1007/s12035-018-1169-7
M.H. Teiten, F. Gaascht, M. Cronauer, E. Henry, M. Dicato, M. Diederich, Anti-proliferative potential of curcumin in androgen-dependent prostate cancer cells occurs through modulation of the wingless signaling pathway. Int. J. Oncol. 38, 603–611 (2011). https://doi.org/10.3892/ijo.2011.905
S.K. Patil, S.A. Patil, M.M. Vadiyar, D.V. Awale, A.S. Sartape, L.S. Walekar, S.S. Kolekar, Tailor-made dicationic ionic liquid as a fluorescent sensor for detection of hydroquinone and catechol. J. Mol. Liq. 244, 39–45 (2017). https://doi.org/10.1016/j.molliq.2017.08.119
P. Bansal, G. Bhanjana, N. Prabhakar, J.S. Dhau, G.R. Chaudhary, Electrochemical sensor based on ZrO2 NPs/Au electrode sensing layer for monitoring hydrazine and catechol in real water samples. J. Mol. Liq. 248, 651–657 (2017). https://doi.org/10.1016/j.molliq.2017.10.098
T.S.K. Naik, B.K. Swamy, Modification of carbon paste electrode by electrochemical polymerization of neutral red and its catalytic capability towards the simultaneous determination of catechol and hydroquinone: a voltammetric study. J. Electroanal. Chem. 804, 78–86 (2017). https://doi.org/10.1016/j.jelechem.2017.08.047
D. Wu, A.C. Sedgwick, T. Gunnlaugsson, E.U. Akkaya, J. Yoon, T.D. James, Fluorescent chemosensors: the past, present and future. Chem. Soc. Rev. 46(23), 7105–7123 (2017). https://doi.org/10.1039/C7CS00240H
A. Kong, N.J. Cox, Allele-sharing models: LOD scores and accurate linkage tests. Am. J. Hum. Genet. 61, 1179–1188 (1997). https://doi.org/10.1086/301592
Y. Xie, C. Zhou, S. Zhang, L. Yan, X. Wu, Y. Shan, A coumarin-based fluorescent probe for the detection of hypochlorite ions and its applications in test paper and cell imaging. ChemistrySelect 5(29), 9240–9244 (2020). https://doi.org/10.1002/slct.202002258
L. Liang, Y. Sun, C. Liu, X. Jiao, Y. Shang, X. Zeng, J. Zhao, Highly selective turn-on fluorescent probe for hypochlorite and viscosity detection. J. Mol. Struct. 1227, 129523 (2021). https://doi.org/10.1016/j.molstruc.2020.129523
B. Zhu, X. Wu, J. Rodrigues, X. Hu, R. Sheng, G.M. Bao, A dual-analytes responsive fluorescent probe for discriminative detection of ClO− and N2H4 in living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 246, 118953 (2021). https://doi.org/10.1016/j.saa.2020.118953
M. Yang, S.C. Lee, M. Kim, M.H. Lim, C. Kim, A multi-functional picolinohydrazide-based chemosensor for colorimetric detection of iron and dual responsive detection of hypochlorite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 245, 118899 (2021). https://doi.org/10.1016/j.saa.2020.118899
K. Ponnuvel, J. Ramamoorthy, G. Sivaraman, V. Padmini, Merocyanine dye-based fluorescent chemosensor for highly selective and sensitive detection of hypochlorous acid and imaging in live cells. ChemistrySelect 3, 91–95 (2018). https://doi.org/10.1002/slct.201701833
Acknowledgements
Authors thank for financial support under DST-IRHPA, FIST, RUSA-MKU and PURSE for instrument facilities.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramamoorthy, J., Sathya, V., Lavanya, R. et al. Highly selective and sensitive response of curcumin thioether derivative for the detection of hypochlorous acid by fluorimetric method. J IRAN CHEM SOC 19, 3327–3335 (2022). https://doi.org/10.1007/s13738-022-02528-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-022-02528-5