Abstract
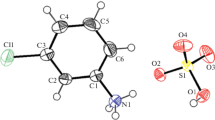
Partially substituted decavanadate in the title compound [NH4][Na2(H2O)10][V9.7W0.3O28] (1) has been obtained by adding ammonium chloride to the acidic reaction mixture. Single-crystal X-ray analysis shows that the ammonium ions pack in a zigzag fashion constructing a ribbon-like structure in the complex along the ac bisector which can be easily intercalated by sodium dimers to form layers. Finally, the last dimension in the hybrid crystalline network is formed by interactions which make the \(R_{4}^{4}\)(22) graph set.




Similar content being viewed by others
References
M. Aureliano, D.C. Crans, J. Inorg. Biochem. 103, 536 (2009)
M. Aureliano, World. J. Biol. Chem. 2, 215 (2011)
S. Sankar Mal, O. Tröppner, I. Ivanović-Burmazović, P. Burger, Eur. J. Inorg. Chem. 11, 1960 (2013)
M. Aureliano, Inorg. Chim. Acta 420, 4 (2014)
M. Aureliano, J. Chem. Soc. Dalton Trans. 9093 (2009)
Y. Hayashi, Coord. Chem. Rev. 255, 2270 (2011)
D. Rehder, Bioinorganic vanadium chemistry, inorganic chemistry: a textbook series (Wiley, New York, 2008)
C. Barriga, W. Jones, P. Malet, V. Rives, M.A. Ulibarri, Inorg. Chem. 37, 1812 (1998)
J. Livage, L. Bouhedja, C. Bonhomme, J. Sol Gel Sci. Tech. 13, 65 (1998)
K. Pavani, S. Upreti, A. Ramanan, J. Chem. Sci. 118, 159 (2006)
R.I. Maksimovskaya, V.M. Bondareva, G.I. Aleshina, Eur. J. Inorg. Chem. 31, 4906 (2008)
U.K. Lee, H.C. Joo, K.M. Park, S. Sankar Mal, U. Kortz, B. Keita, L. Nadjo, Angew. Chem. Int. Ed. 47, 793 (2008)
N. Pavlovic, J. Prevost, A. Spasojevic-de Bire, Cryst. Growth Des. 11, 3778 (2011)
S. Konaka, Y. Ozawa, T. Shonaka, S. Watanabe, A. Yagasaki, Inorg. Chem. 50, 6183 (2011)
F. Yao, Y.G. Chen, A.R. Salimi, M. Mirzaei, J. Clust. Sci. 22, 309 (2011)
H. Eshtiagh-Hosseini, M. Mirzaei, J. Clust. Sci. 23, 345 (2012)
Bruker-AXS, APEX2, SAINT, SADABS and SHELXT (Madison, 2014)
G.M. Sheldrick, Acta Cryst. C71, 3 (2015)
I. Mestiri, B. Ayed, A. Haddad, J. Clust. Sci. 24, 85 (2013)
Acknowledgments
MM thanks the financial support by the Ferdowsi University of Mashhad. JTM thanks Tulane University for support of the Tulane Crystallography Laboratory.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Najafi, A., Mague, J.T. & Mirzaei, M. Non-covalent interactions in tungsten-doped sodium ammonium decavanadate decahydrate. J IRAN CHEM SOC 13, 773–777 (2016). https://doi.org/10.1007/s13738-015-0790-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0790-x