Abstract
We present three cases of IgA nephropathy with gross hematuria following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger RNA (mRNA) vaccination. Case 1 was a 60-year-old woman who has previously experienced transient proteinuria. Case 2 was a 22-year-old woman with no history of urinary abnormality. Finally, case 3 was a 66-year-old woman who has had microscopic hematuria since she was in her 50s. They were all diagnosed as IgA nephropathy with little histological active lesion. Their renal function and proteinuria improved without the use of corticosteroids. There were differences in the findings of vascular endothelial damage based on the time between the appearance of gross hematuria and renal biopsy. Glomerular endocapillary damage could be a part of the mechanism triggered by mRNA vaccination. When a patient presents with gross hematuria following vaccination, a comprehensive approach including renal biopsy should be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Given the global pandemic of the new coronavirus disease 2019, vaccine development and practical application are underway. In Japan, messenger RNA (mRNA) vaccines such as Pfizer BNT162b2 and Takeda/Moderna mRNA-1273, as well as virus vector vaccine such as AstraZeneca AZD1222, are currently in use.
Several reports on cases of gross hematuria following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccination, particularly those with IgA nephropathy, have been published [1]. A case of newly diagnosed IgA nephropathy with no known glomerulonephritis history has also been reported [2]. According to a questionnaire survey of councilor members conducted by the Japanese Society of Nephrology, the majority of cases did not progress to serious renal dysfunction. However, several cases of rapidly progressive glomerulonephritis with significant renal dysfunction have been reported worldwide. It is critical to investigate the safety of the vaccination in patients with chronic glomerulonephritis, particularly IgA nephropathy, as well as the treatment strategy for patients who develop urinary abnormalities after vaccination.
Here, we present three cases of IgA nephropathy diagnosed by renal biopsy who presented with gross hematuria after SARS-CoV-2 mRNA vaccination, along with their renal pathology findings and clinical courses.
Case report
Case 1
A 60-year-old woman was referred to our department for severe hematuria following the SARS-CoV-2 mRNA vaccination. She had a history of transient proteinuria from 15 to 19 years old. A renal biopsy was not performed. She was being treated of hypertension with olmesartan and amlodipine. On July 1, 2020, her serum creatinine (Cre) level was 0.82 mg/dL. She received her first dose of mRNA vaccine (Pfizer BNT162b2) on June 14, 2021. On July 2, her regular medical checkup revealed a Cre level of 0.97 mg/dL, as well as a positive urine protein and urine occult blood. On July 5, the second vaccine (the same as the first) was administered. On July 6, she developed a fever and gross hematuria. On July 8, the patient was referred to our hospital. Urinalysis at the time of the first visit revealed urinary red blood cells (U-RBC) > 100/high-power field (HPF), proteinuria of 0.54 g/gCre (urine protein-to-creatinine ratio [UPCR]), and mildly elevated Cre level at 1.10 mg/dL. Although the gross hematuria disappeared in 2 days, the microscopic hematuria, proteinuria, and renal dysfunction persisted on July 14.
Laboratory examination showed serum Cre 0.96 mg/dL, estimated glomerular filtration rate (eGFR) 46.3 mL/min/1.73 m2, cystatin C 0.910 mg/L, UPCR 0.28 g/gCr, U-RBC 1–4/HPF (other results are summarized in Table 1).
On July 28, 3 weeks after the onset of gross hematuria, a renal biopsy was performed. Light microscopy (LM) revealed a total of 27 glomeruli, 6 of which were globally sclerotic. The remainder had mild diffuse mesangial hypercellularity (Fig. 1). One had glomerular tuft segmental collapse and a small infiltrate of polynuclear leukocytes and monocytes. Endocapillary hypercellularity was present in one. Immunofluorescence (IF) analysis revealed mesangial deposition of IgA, C3, and IgM. There were electron-dense deposits (EDDs) in the mesangial and paramesangial areas in electron microscopy (EM). We identified IgA nephropathy, with an Oxford MEST-C score of M0E1S1T0C0. In 4 weeks, urine protein decreased and became negative. Microscopic hematuria disappeared 4 months after the gross hematuria episode. Renal function remained unchanged. We continued blood pressure control with renin–angiotensin system inhibitors and regular checkups.
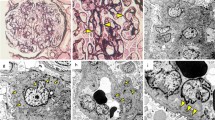
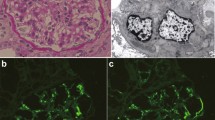
A renal biopsy sample of case 1. a Light microscopy showing mild diffuse mesangial hypercellularity. This glomerulus exhibits segmental sclerotic lesion and segmental endocapillary hypercellularity with polynuclear cells (Masson’s trichrome stain, × 400). b Immunofluorescence showing granular staining for IgA in the mesangium. (FITC anti-human IgA, × 400). c Electron microscopy showing findings of subendothelial space enlargement and endothelial cell swelling (red allow) (× 1500). d Enlarged view of the area squared in red in Fig. 1c. The red arrow indicates subendothelial space enlargement and endothelial cell swelling (× 6000). e Electron microscopy showing electron-dense deposits in the mesangial region (red arrow head) (× 5000)
Case 2
A 22-year-old woman, with a history of tonsillitis when she was in school, but had never mentioned an abnormality in her urine during school or workplace checkups, experienced gross hematuria after vaccination. The first dose of mRNA vaccine (Moderna mRNA-1273) was given on August 14, 2021, and the second dose (same as the first) was given on September 11. She had a fever of 39 °C on the day of the vaccination, and had gross hematuria 2 days later. On September 13, she visited her local doctor. At the first visit, urine occult blood 3 + , urine protein 3 + , and a repeated examination on September 21 showed the same results. The patient was referred to our department on October 14. At the time of referral, urinalysis showed U-RBC 10–19/HPF and UPCR 0.04 g/gCr.
Blood examination showed Cre 0.67 mg/dL, eGFR 91.5 mL/min/1.73 m2, cystatin C 0.76 mg/L. Urinalysis showed UPCR 0.04 g/gCr, U-RBC 10–19/HPF, 24 h CCr 132.0 mL/min (Table 1).
Six weeks after the onset of gross hematuria, renal biopsy was performed. LM revealed 54 glomeruli, with no sclerotic glomeruli. There were mild mesangial cell proliferation and mesangial matrix expansion. The adhesion of glomerular capillary to Bowman's capsule was observed in a glomerulus. There was no evidence of interstitial fibrosis or tubular atrophy. IF revealed IgA and C3 mesangial deposition. In EM, EDDs were observed in the mesangial area. The subendothelial space was slightly enlarged, and endothelial cells swelled (Fig. 2).
A renal biopsy sample of case 2. a Light microscopy showing mild mesangial cell proliferation and mesangial matrix expansion (PAS, × 400). b Light microscopy showing a glomerulus with adhesion of glomerular capillary to Bowman’s capsule. (PAS, × 400). c Immunofluorescence showing granular staining for IgA in the mesangium. (FITC anti-human IgA, × 400). d Electron microscopy showing electron-dense deposits in the mesangial region (red allow head). The red arrow indicates slight subendothelial space enlargement and endothelial cell swelling (× 6000)
By the time the renal biopsy was performed, the patient's renal function had already recovered. The renal biopsy revealed IgA nephropathy with an Oxford MEST-C classification of M0S0E0T0C0 and no active lesions. We decided to continue with outpatient care without steroid therapy.
Case 3
A 66-year-old woman, who has been on atorvastatin for dyslipidemia, was referred to our hospital for hematuria. She had been diagnosed with microscopic hematuria without proteinuria at medical checkups since her 50 s. Her renal function was Cre 0.66 mg/dL in 2020. On May 30, 2021, the first dose of mRNA vaccine (Pfizer BNT162b2) was administered. The second vaccine (same as the first) was administered on June 21. Fever and gross hematuria were observed on the night of the inoculation. Gross hematuria cleared up in about a week, but microscopic hematuria persisted, and the patient was referred to our hospital's urology department on August 26. There were no obvious urological abnormalities. On September 2, she was referred to our department. Urine and blood examination revealed U-RBC > 100/HPF, UPCR 0.18 g/gCre, and Cre 0.83 mg/dL. Chronic glomerulonephritis was suspected and a renal biopsy was performed on October 19, four months after the onset of gross hematuria.
Blood examination just before the biopsy revealed Cre 0.77 mg/dL, eGFR 57.3 mL/min/1.73 m2, cystatin C 1.020 mg/L. Urinalysis showed UPCR 0.22 g/gCr, U-RBC 1–4/HPF, 24 h CCr 81.9 mL/min (Table 1).
LM revealed eight glomeruli in total, with one completely sclerotic glomerulus. There was no mesangial cell proliferation, nor was there an increase in mesangial matrix or endocapillary proliferation (Fig. 3). In 8% of the tubules, there was atrophy, and in eight locations, there was mild to moderate focal lymphocytic infiltration. Interlobular arteries showed mild atherosclerosis. IF revealed mesangial deposition of IgA, C3, and IgM. In EM, EDDs were found in the mesangial and paramesangial areas.
A renal biopsy sample of case 3. a Light microscopy showing no mesangial cell proliferation, nor an increase in mesangial matrix or endocapillary proliferation (PAS, × 400). b Immunofluorescence showing granular staining for IgA in the mesangium. (FITC anti-human IgA, × 400). c Electron microscopy showing electron-dense deposits in the mesangial region (red allow head). There has been no evidence of vascular endothelial damage (× 1500)
Histological findings were consistent with IgA nephropathy, with an Oxford MEST-C classification of M0S0E0T0C0. The patient was treated conservatively with diet therapy (salt restriction) and regular follow-up, and renal function recovered to baseline with trivial urinary protein.
Discussion
We presented three cases of IgA nephropathy diagnosed by renal biopsy after gross hematuria following SARS-CoV-2 mRNA vaccination. After the second vaccination, all cases were female and had severe hematuria. They all had a mild increase in Cre level after the onset of hematuria, but none had a significant decline in renal function. These findings are consistent with the report by the Japanese Society of Nephrology [3].
Following SARS-CoV-2 mRNA vaccination, several cases of nephritis have been reported, including IgA nephropathy, minimal change nephrotic syndrome, membranous nephropathy, focal segmental glomerulosclerosis, MPO-ANCA associated vasculitis, and anti-GBM disease. According to a web-based survey conducted by the Japanese Society of Nephrology, there were 27 cases of gross hematuria after vaccination, with 19 (70.4%) cases already diagnosed as IgA nephropathy. As far as the literature could be searched in January 2022, there were 18 reported IgA nephropathy cases of gross hematuria after SARS-CoV-2 vaccination that could be confirmed with individual histories (Table 2).
Including our three cases, there were 21 cases who presented with gross hematuria following SARS-CoV-2 vaccination. Following vaccination, all of the patients in our study were diagnosed with IgA nephropathy based on renal biopsy findings. Eleven of the 21 cases were newly diagnosed with IgA nephropathy, whereas the remaining 10 were previously confirmed cases of IgA nephropathy. The patients in our three cases were referred to our department due to severe hematuria, which resulted in a new diagnosis of IgA nephropathy. However, the clinical courses of cases 1 and 3 have previously included proteinuria or microscopic hematuria, implying the possibility of chronic glomerulonephritis. It is possible that the immune activation associated with vaccination exacerbated the latent IgA nephropathy.
Proteinuria was observed in case 1 for several years during childhood, and a chronic glomerulonephritis may have been present during this time. Histopathology of the kidney revealed a very narrowly confined tubulointerstitial fibrosis with only one site of segmental sclerosis. It is difficult to say whether or not the segmental sclerosis occurred after the first vaccination. Four of the six global sclerosis were found in the glomerulus just beneath the capsule and are thought to be non-specific sclerosis unrelated to the nephritis. Based on these findings, it is unlikely that the nephritis progressed slowly over a long period. On the other hand, EM reveals findings of subendothelial space enlargement and endothelial cell swelling, indicating vascular endothelial damage. Because the biopsy was performed approximately 3 weeks after the second vaccination, it is possible that the immune response caused the endothelial damage. Kudose et al. reported two cases of gross hematuria with renal biopsy findings performed within 3 weeks of vaccination. IgA nephropathy with endocapillary hypercellularity was seen in both cases [4].
Light microscopic findings of Case 2, which was performed 6 weeks after the second vaccination, suggested low activity of glomerulonephritis, but EM revealed enlargement of the subendothelial space and endothelial cell swelling.
In case 3, the nephritis was not active, and age-related atherosclerotic changes were prominent. In contrast to case 1, there was no evidence of vascular endothelial damage. In case 3, the biopsy was performed 4 months after the second vaccination. Although we cannot deny the possibility that endothelial damage findings similar to those in case 1 appeared during the course of the disease, there were no crescent formation findings. Even though this study is limited to three cases and should not be considered conclusive, it is possible that vaccination causes endothelial damage in the glomerular capillary, which can persist for several weeks.
Patients with IgA nephropathy frequently present with gross hematuria following upper respiratory tract infection or enteritis. In addition to gross hematuria, increased proteinuria and renal dysfunction may occur. It is debatable whether the worsening of nephritis symptoms caused by infection is the same as the renal pathology after SARS-CoV2 mRNA vaccination. This necessitates further research into the immune mechanisms of mRNA vaccination as well as the immune mechanisms of IgA nephropathy. Gross hematuria following Adenovirus vector vaccine or inactivated vaccine has not yet been reported.
The nucleoside modified, purified mRNA lipid nanoparticle-encapsulated platform is used in the BNT162b2 (Pfizer) and the mRNA-1273 (Moderna) vaccines. This novel RNA platform induces a stronger antigen-specific cluster of differentiation (CD) 4 + and CD8 + T cell responses in experimental animals [5]. When activated by mRNA vaccines, CD4 + and CD8 + T cells produce a variety of proinflammatory cytokines, including interferon-γ and tumor necrosis factor-α, which may activate or exacerbate immune-mediated glomerular diseases [6].
Toll-like receptors (TLRs) are thought to play a role in the pathogenesis of IgA nephropathy. It is well known that IgA1 extracted from serum and glomeruli of patients with IgA nephropathy contains abnormally glycosylated IgA1. TLR9, which recognizes single-stranded DNA with unmethylated CpG motifs, has been linked to the synthesis of this IgA [7]. On the other hand, it has been reported that TLR7, which recognizes endogenous or exogenous single-stranded RNA, is also involved in the production of abnormally glycosylated IgA1 [8]. As a result, mRNA vaccination may lead to the production of abnormally glycosylated IgA1 via the TLR signaling system.
Reports from hospitalized COVID-19 patients revealed an activation of the immune system. Hyper-inflammatory state has deleterious effects on the vascular system resulting in endothelial cell dysfunction. In the presence of circulating inflammatory mediators such as interleukin (IL)-1, IL-6, damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs), endothelial cells undergo transition to activated status [9]. Activated endothelial cells promote localized inflammation by inducing proinflammatory gene expression, attracting immune cells, promoting recruitment of inflammatory cells to injured or infected tissues, vascular leak by increasing endothelial permeability, and altering the thrombotic potential of the local intimal surface. Additionally, endothelial cell injury is compounded by TLR activation by viral RNA recognition, resulting increased reactive oxygen species (ROS) production [10]. These findings suggest that, as with SARS-COV-2 infections, vaccination could lead to vascular endothelial damage.
In our study, there were no cases of acute kidney injury (AKI). According to the findings of a questionnaire survey conducted by the Japanese Society of Nephrology on the relationship between SARS-CoV-2 vaccination and the emergence of gross hematuria, only one case of gross hematuria after vaccination resulted in AKI. On the other hand, there are several reports of AKI cases worldwide, and many of these cases show a worsening of proteinuria (Table 2). All patients in our study had IgA nephropathy, had little histological active disease, and had improved renal function and proteinuria with conservative treatment. On the other hand, globally, there have been reports of cases with gross hematuria after SARS-CoV-2 mRNA vaccination who also had a severe clinical course (rapidly progressive glomerular nephritis, crescent formation) in anti-GBM disease and IgA nephropathy. It is necessary to recognize that there may be such a deteriorating case [4, 11,12,13].
In conclusion, although vaccination is an important strategy for effectively controlling the spread of SARS-CoV-2 infections, mRNA vaccine could trigger activation of glomerulonephritis through endothelial damages. When a patient presents with gross hematuria following vaccination, a renal biopsy should be performed, and a comprehensive approach should be considered based on histopathological findings and clinical course.
References
Abramson M, Mon-Wei YuS, Campbell KN, Chung M, Salem F. IgA nephropathy after SARS-Cov-2 vaccination. Kidney Med. 2021;3:860–3.
Klomjit N, Alexander MP, Fervenza FC, et al. COVID-19 vaccination and glomerulonephritis. Kidney Int Rep. 2021;6:2969–78.
Matsuzaki K, Aoki R, Nihei Y, Suzuki H, Kihara M, Yokoo T, Kashihara N, Narita I, Suzuki Y. Gross hematuria after SARS-CoV-2 vaccination: questionnaire survey in Japan. Clin Exp Nephrol. 2021;13:1–7.
Kudose S, Friedmann P, Albajrami O, D’Agati VD. Histologic correlates of gross hematuria following Moderna COVID-19 vaccine in patients with IgA nephropathy. Kidney Int. 2021;100:468–9.
Pardi N, Hogan MJ, Naradikian MS, et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018;215:1571–88.
Negrea L, Rovin BH. Gross hematuria following vaccination for severe acute respiratory syndrome coronavirus 2 in 2 patients with IgA nephropathy. Kidney Int. 2021;99:1487.
Makita Y, Suzuki H, Kano T, et al. TLR9 activation induces aberrant IgA glycosylation via APRIL- and IL-6-mediated pathways in IgA nephropathy. Kidney Int. 2020;97:340–9.
Zheng N, Xie K, Ye H, et al. TLR7 in B cells promotes renal inflammation and Gd-IgA1 synthesis in IgA nephropathy. JCI Insight. 2020;5: e136965.
Pons S, Arnaud M, Loiselle M, Arrii E, Azoulay E, Zafrani L. Immune consequences of endothelial cells activation and dysfunction during sepsis. Crit Care Clin. 2020;36(2):401–13.
To EE, Vlahos R, Luong R, Halls ML, Reading PC, King PT, et al. Endosomal NOX2 oxidase exacerbates virus pathogenicity and is a target for antiviral therapy. Nat Commun. 2017;8(1):69.
Tan HZ, Tan RY, Choo JCJ, et al. Is COVID-19 vaccination unmasking glomerulonephritis? Kidney Int. 2021;100:469–71.
Sacker A, Kung V, Andeen N. Anti-GBM nephritis with mesangial IgA deposits after SARS-CoV-2 mRNA vaccination. Kidney Int. 2021;100:471–2.
Hanna C, Herrera Hernandez LP, Bu L, et al. IgA nephropathy presenting as macroscopic hematuria in 2 pediatric patients after receiving the Pfizer COVID-19 vaccine. Kidney Int. 2021;100:705–6.
Rahim SEG, Lin JT, Wang JC. A case of gross hematuria and IgA nephropathy fare-up following SARS-CoV-2 vaccination. Kidney Int. 2021;100:238.
Perrin P, Bassand X, Benotmane I, Bouvier N. Gross hematuria following SARS-CoV-2 vaccination in patients with IgA nephropathy. Kidney Int. 2021;100:466–8.
Plasse R, Nee R, Gao S, Olson S. Acute kidney injury with gross hematuria and IgA nephropathy after COVID-19 vaccination. Kidney Int. 2021;100:944–5.
Fujita Y, Yoshida K, Ichikawa D, Shibagaki Y, Yazawa M. Abrupt worsening of occult IgA nephropathy after the first dose of SARS-CoV-2 vaccination. CEN Case Rep. 2022;6:1–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest associated with this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent were obtained from all the patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ota, K., Yonekura, Y., Saigan, M. et al. Comparison of renal histopathology in three patients with gross hematuria after SARS-CoV-2 vaccination. CEN Case Rep 12, 176–183 (2023). https://doi.org/10.1007/s13730-022-00743-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-022-00743-w