Abstract
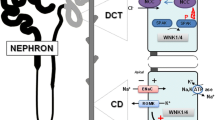
Owing to its rarity and severe nature, the treatment for generalized pseudohypoaldosteronism type 1 (PHA1), a genetic disorder in the epithelial sodium channel (ENaC), is exclusively experience-based. In particular, the usefulness of dietary potassium restriction in PHA1 remains unclear with the absence of theoretical background to elucidate its utility. First, we demonstrated the effect of potassium restriction in a 13-month-old patient with ENaC γ-subunit gene mutations via a retrospective chart review; reduction of daily dietary potassium intake from 40 to 20 mEq induced rapid restoration of volume depletion, as evidenced by weight gain, elevation of the serum sodium level from 133 to 141 mEq/L, decreased urinary sodium excretion, and normalized renin activity. The serum potassium level decreased from 5.6 to 4.5 mEq/L. Next, we attempted to elucidate the pathophysiological basis of the usefulness of potassium restriction, leveraged by the increased knowledge regarding the roles of with-no-lysine kinases (WNKs) in the distal nephron. When potassium is restricted, the WNK signal will turn “on” in the distal nephron via reduction in the intracellular chloride level. Consequently, the sodium reabsorption from the Na+Cl− cotransporter (NCC) in the distal convoluted tubule and possibly from pendrin in the β-intercalated cell will increase. Thus, potassium restriction causes NCC and pendrin to compensate for the non-functional ENaC in the collecting duct. In conclusion, dietary potassium restriction is one of the indispensable treatments for generalized PHA1.


Similar content being viewed by others
Change history
29 April 2020
In the Original publication of the article, there are two minor errors in Fig.��2 and these include one missing arrow in Fig.��2d and appears as an incorrectly drawn solid lines as dashed line in Fig.��2d.
References
Zennaro MC, Hubert EL, Fernandes-Rosa FL. Aldosterone resistance: structural and functional considerations and new perspectives. Mol Cell Endocrinol. 2012;350:206–15.
Riepe FG. Pseudohypoaldosteronism. Endocr Dev. 2013;24:86–95.
Hanukoglu I, Hanukoglu A. Epithelial sodium channel (ENaC) family: phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene. 2016;579:95–132.
Tajima T, Morikawa S, Nakamura A. Clinical features and molecular basis of pseudohypoaldosteronism type 1. Clin Pediatr Endocrinol. 2017;26:109–17.
Adachi M, Tachibana K, Asakura Y, Abe S, Nakae J, Tajima T, Fujieda K. Compound heterozygous mutations in the gamma subunit gene of ENaC (1627delG and 1570-1G %3e A) in one sporadic Japanese patient with a systemic form of pseudohypoaldosteronism type 1. J Clin Endocrinol Metab. 2001;86:9–12.
Hanukoglu I, Boggula VR, Vaknine H, Sharma S, Kleyman T, Hanukoglu A. Expression of epithelial sodium channel (ENaC) and CFTR in the human epidermis and epidermal appendages. Histochem Cell Biol. 2017;147:733–48.
Kerem E, Bistritzer T, Hanukoglu A, Hofmann T, Zhou Z, Bennett W, MacLaughlin E, Barker P, Nash M, Quittell L, Boucher R, Knowles MR. Pulmonary epithelial sodium-channel dysfunction and excess airway liquid in pseudohypoaldosteronism. N Engl J Med. 1999;341:156–62.
Güran T, Değirmenci S, Bulut İK, Say A, Riepe FG, Güran Ö. Critical points in the management of pseudohypoaldosteronism type 1. J Clin Res Pediatr Endocrinol. 2011;3:98–100.
Adachi M, Asakura Y, Muroya K, Tajima T, Fujieda K, Kuribayashi E, Uchida S. Increased Na reabsorption via the Na-Cl cotransporter in autosomal recessive pseudohypoaldosteronism. Clin Exp Nephrol. 2010;14:228–32.
Fanelli F, Baronio F, Ortolano R, Mezzullo M, Cassio A, Pagotto U, Balsamo A. Normative basal values of hormones and proteins of gonadal and adrenal functions from birth to adulthood. Sex Dev. 2018;12:50–94.
Argaiz ER, Gamba G. The regulation of Na+Cl− cotransporter by with-no-lysine kinase 4. Curr Opin Nephrol Hypertens. 2016;25:417–23.
Hadchouel J, Ellison DH, Gamba G. Regulation of renal electrolyte transport by WNK and SPAK-OSR1 kinases. Annu Rev Physiol. 2016;78:367–89.
van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJ. K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl- cotransporter. Am J Physiol Renal Physiol. 2013;305:F1177–F1188188.
Welling PA. Regulation of renal potassium secretion: molecular mechanisms. Semin Nephrol. 2013;33:215–28.
Naito S, Ohta A, Sohara E, Ohta E, Rai T, Sasaki S, Uchida S. Regulation of WNK1 kinase by extracellular potassium. Clin Exp Nephrol. 2011;15:195–202.
Arroyo JP, Ronzaud C, Lagnaz D, Staub O, Gamba G. Aldosterone paradox: differential regulation of ion transport in distal nephron. Physiology (Bethesda). 2011;26:115–23.
López-Cayuqueo KI, Chavez-Canales M, Pillot A, Houillier P, Jayat M, Baraka-Vidot J, Trepiccione F, Baudrie V, Büsst C, Soukaseum C, Kumai Y, Jeunemaître X, Hadchouel J, Eladari D, Chambrey R. A mouse model of pseudohypoaldosteronism type II reveals a novel mechanism of renal tubular acidosis. Kidney Int. 2018;94:514–23.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in this study were in accordance with the ethical standards of national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was in accordance with the ethical standards of Kanagawa Children’s Medical Center.
Informed consent
Informed consent was obtained from the patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Adachi, M., Tajima, T. & Muroya, K. Dietary potassium restriction attenuates urinary sodium wasting in the generalized form of pseudohypoaldosteronism type 1. CEN Case Rep 9, 133–137 (2020). https://doi.org/10.1007/s13730-019-00441-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-019-00441-0