Abstract
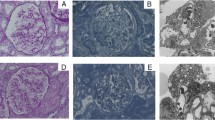
Fabry disease is an X-linked lysosomal storage disorder caused by a lack of α-galactosidase A activity, which leads to the accumulation of globotriaosylceramide in various organs. A complete lack of α-galactosidase A activity in a hemizygous male is the classical phenotype, and some hemizygous males show primarily cardiac and/or renal symptoms that appear in adulthood; this is called the variant type or the late-onset type. The kidney and heart are the major target organs, with damage to these organs related to mortality. Thus, in Fabry patients, early detection and early treatment are critical to longevity. Here, we present a 55-year-old Japanese male patient who was diagnosed with late-onset Fabry nephropathy with cardiomyopathy but with no abnormal urinary findings except for urinary mulberry cells and mulberry bodies. In spite of the absence of abnormal urinary findings, the light microscopic and electron microscopic pathological findings showed extensive deposition of globotriaosylceramide to podocytes. In this paper, we propose that the presence of mulberry cells and mulberry bodies can be used for the earlier detection of Fabry nephropathy, especially the late-onset type.



Similar content being viewed by others
References
Kint JA. Fabry’s disease: alpha-galactosidase deficiency. Science. 1970;167:1268–9.
Tondel C, Bostad L, Larsen KK, et al. Agalsidase benefits renal histology in young patients with Fabry disease. J Am Soc Nephrol. 2013;24:137–8.
Pisani A, Visciano B, Imbriaco M, et al. The kidney in Fabry’s disease. Clin Genet. 2014;86:301–9.
Terryn W, Cochat P, Froissart R, et al. Fabry nephropathy: indications for screening and guidance for diagnosis and treatment by the European Renal Best Practice. Nephrol Dial Transplant. 2013;28:505–17.
Ortiz A, Oliveira JP, Wanner C, Brenner BM, Waldek S, Warnock DG. Recommendations and guidelines for the diagnosis and treatment of Fabry nephropathy in adults. Nat Clin Pract Nephrol. 2008;4:327–36.
Nakao S, Takenaka T, Maeda M, et al. An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N Engl J Med. 1995;333:288–93.
Nakao S, Kodama C, Takenaka T, et al. Fabry disease: detection of undiagnosed hemodialysis patients and identification of a “renal variant” phenotype. Kidney Int. 2003;64:801–7.
Tondel C, Kanai T, Larsen KK, et al. Foot process effacement is an early marker of nephropathy in young classic Fabry patients without albuminuria. Nephron. 2015;129:16–21.
Eng CE, Fletcher J, Wilcox WR, et al. Fabry disease: baseline medical characteristics of a cohort of 1765 males and females in the Fabry Registry. J Inherit Metab Dis. 2007;30:182.
Trimarchi H, Canzonieri R, Muryan A, et al. Copious podocyturia without proteinuria and with normal renal function in a young adult with Fabry disease. Case Rep Nephrol. 2015;257628.
Becher GJ, Nicholls K. Lipiduria-with special relevance to Fabry disease. Clin Chem Lab Med. 2015;53:s1465–70.
Nakamichi T, Miyazaki M, Nakayama K, et al. Fabry’s disease discovered with chance urinary mulberry cells: a case report. CEN Case Rep. 2013;2:49–52.
Honda T, Komatsu E, Furuse S, Mise N. Fabry disease diagnosed based on the detection of urinary mulberry bodies. Int Med. 2016;55:2903.
Shimohata H, Ogawa Y, Maruyama H, Hirayama K, Kobayashi M. A case of renal variant of Fabry disease diagnosed by the presence of urinary mulberry cells. Int Med. 2016;55:3475–8.
Najafian B, Svarstad E, Bostad L, et al. Progressive podocyte injury and globotriaosylceramide (Gb-3) accumulation in young patients with Fabry disease. Kidney Int. 2010;79:663–70.
Tondel C, Bostad L, Hirth A, Svarstad E. Renal biopsy findings in children and adolescents with Fabry disease and minimal albuminuria. Am J Kidney Dis. 2008;51:767–76.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Human and animal rights
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
About this article
Cite this article
Shimohata, H., Maruyama, H., Miyamoto, Y. et al. Urinary mulberry cells and mulberry bodies are useful tool to detect late-onset Fabry disease. CEN Case Rep 6, 148–151 (2017). https://doi.org/10.1007/s13730-017-0262-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-017-0262-5