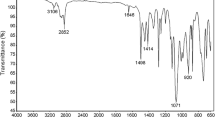
Abstract
A series of well-defined acrylamide/methyl methacrylate random copolymers (PAM-ran-PMMA) were synthesized in this work. The polymerization reactions were carried out in N,N-dimethylformamide (DMF) at 25 °C using Cu(0)/hexamethylenetetramine (HMTA) and CCl4/hydrazine as the catalyst system and the initiator system, respectively. The number average molecular weights (Mn) and the distribution of molecular weight (Mw/Mn) of copolymers were analyzed by gel permeation chromatography (GPC). Experimental results revealed that the copolymerization reaction follows a pseudo first-order kinetic model. Mn of PAM-ran-PMMA increased linearly with the conversion of monomers while a narrow molecular weight distribution was obtained. By means of Fineman–Ross equation, the reactivity ratios of r1 (AM) and r2 (MMA) were calculated to be 0.81 and 3.21, respectively. The results implied that the amount of AM unites in PAM-ran-PMMA copolymers increased with the increase of the molar ratios of AM/MMA. The structure and the thermal stability of the resultant PAM-ran-PMMA copolymer were characterized by Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance spectrometry (1H NMR) and thermogravimetric analysis (TGA). TGA revealed that copolymers with a greater content of AM unit exhibited a higher thermal stability. The obtained PAM-ran-PMMA copolymer was used as a macroinitiator to perform Cu(0)-catalyzed chain extension experiment, which led to the increase of Mn and demonstrated the living character of the polymerization. This current approach provided a controlled route for the synthesis of well-defined PAM-ran-PMMA.
Graphical abstract







Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
NothlingMD CaoH, McKenzie TG, Hocking DM, Strugnell RA, Qiao GG (2021) Bacterial redox potential powers controlled radical polymerization. J Am Chem Soc 143:286–293
Azuma Y, Terashima T, Sawamoto M (2017) Self-folding polymer iron catalysts for living radical polymerization. ACS Macro Lett 6:830–835
OliverS ZL, Gormley AJ, Chapman R, Boyer C (2019) Living in the fast lane-high throughput controlled/living radical polymerization. Macromolecules 52:3–23
Dervaux B, Camp WV, Du Prez FE (2008) Amphiphilic block and “block and like” copolymers based on poly(isobornyl acrylate) and poly(acrylic acid) via ATRP. Polym Prep 49:6–7
Lyra EP, Petzhold CL, Lona LMF (2019) Tin(II) 2-ethylhexanoate and ascorbic acid as reducing agents in solution ARGET ATRP: a kinetic study approach by mathematical modeling and simulation. Chem Eng J 364:186–200
Zhou Y, Wang K, Hu D (2021) An aqueous approach to functionalize waterlogged archaeological wood followed by improved surface-initiated ARGET ATRP for maintaining dimensional stability. Cellulose 28:2433–2443
Min K, Gao H, Matyjaszewski K (2007) Use of ascorbic acid as reducing agent for synthesis of well-defined polymers by ARGET ATRP. Macromolecules 40:1789–1791
Karkare P, Kumar S, Murthy CN (2019) ARGET-ATRP using β-CD as reducing agent for the synthesis of PMMA-b-PS-b-PMMA triblock copolymers. J Appl Polym Sci 136:47117
Paterson SM, Brown DH, Chirila TV, Keen I, Whittaker AK, Baker MV (2010) The synthesis of water-soluble PHEMA via ARGET ATRP in protic media. J Polym Sci 48:4084–4092
Kwak Y, Magenau AJD, Matyjaszewski K (2011) ARGET ATRP of methyl acrylate with inexpensive ligands and ppm concentrations of catalyst. Macromolecules 44:811–819
Leophairatana P, Samanta S, De Silva CC, Koberstein JT (2017) Preventing alkyne-alkyne (i.e., Glaser) coupling associated with the ATRP synthesis of alkyne-functional polymers/macromonomers and for alkynes under click (i.e., CuAAC) reaction conditions. J Am Chem Soc 139:3756–3766
Li Z, Shi S, Fei Y, Cao D, Zhang K, Wang B, Zhe Ma, Li P, Li Y (2020) Supertough and transparent poly(lactic acid) nanostructure blends with minimal stiffness loss. ACSOmega 5:13148–13157
Wu X, Chen X, Shi L, Fan Z (2016) Preparation, structure and properties of PLLA-TMC/PDLA-TMC stereocomplexes. Chem J Chin Univ 37:2101–2107
Zhong Z, Wang X, Zhao S, Peng F, Wang J, Ying L, Yang W, Peng J, Cao Y (2017) Effects of a random copolymer’s component distribution on its opto-electronic properties. J Mater Chem C 5:6163–6168
Tévenot Q, Kawahara S (2021) ATRP-ARGET of a styrene monomer onto modified natural rubber latex as an initiator. Langmuir 37:6151–6157
Cao J, Song T, Zhu Y, Wang S, Wang X, Lv F, Jiang L, Sun M (2018) Application of amino-functionalized nanosilica in improving the thermal stability of acrylamide-based polymer for enhanced oil recovery. Energy Fuel 32:246–254
Teh CY, Budiman PM, Shak KPY, Wu TY (2016) Recent advancement of coagulation-flocculation and its application in wastewater treatment. Ind Eng Chem Res 55:4363–4389
Alsubaie FM, Alothman OY, Alshammari BA, Fouad H (2021) Facile synthesis of hydrophilic homo-polyacrylamides via Cu(0)-mediated reversible deactivation radical polymerization. Polymers 13:1947–1958
Fan Y, Cao H, van Mastrigt F, Pei Y, Picchioni F (2018) Copper-mediated homogeneous living radical polymerization of acrylamide with waxy potato starch-based macroinitiator. Carbohyd polym 192:61–68
Abdurrahmanoglu S, Can V, Okay O (2009) Design of high-toughness polyacrylamide hydrogels by hydrophobic modification. Polymer 50:5449–5455
Wu S, Shanks RA (2004) Synthesis and characterization of hydrophobic modified polyacrylamide. Polym Int 53:1821–1830
Zhou Y, Zheng H, Huang Y, Zheng X, Liu Z, An Y, Liu Y (2019) Hydrophobic modification of cationic microblocked polyacrylamide and its enhanced flocculation performance for oily wastewater treatment. J Mater Sci 54:10024–10040
Zhuang L, Zhi X, Du B, Yuan S (2020) Preparation of elastic and antibacterial chitosan-citric membranes with high oxygen barrier ability by in situ cross-linking. ACS Omega 5:1086–1097
Hsiao S-H, Wang HM (2016) Facile fabrication of redox-active and electrochromic poly(amide-amine) films through electrochemical oxidative coupling of arylamino groups. Polym Chem 54:2476–2485
Zhang H, Carrillo-Navarrete F, López-Mesas M, Palet C (2020) Use of chemically treated human hair wastes for the removal of heavy metal ions. Water 12:1263–1273
Oualid HA, Abdellaoui Y, Laabd M, Ouardi ME, Brahmi Y, Iazza M, Oualid JA (2020) Eco-efficient green seaweed codium decorticatum biosorbent for textile dyes: characterization, mechanism, recyclability, and RSM optimization. ASC Omega 5:22192–22207
Song YY, Dong B, Wang SW, Wang ZR, Zhang M, Tian P, Wang GC, Zhao Z (2020) Selective oxidation of propylene on Cu2O(111) and Cu2O(110) surfaces: a systematically DFT study. ACS Omega 5:6260–6269
Andal V, Buvaneswari G (2017) Effect of reducing agents in the conversion of Cu2O nanocolloid to Cu nanocolloid. Eng Sci Technol 20:340–344
Fineman M, Ross SD (1950) Linear method for determining monomer reactivity ratios in copolymerization. J Polym Sci 5:259–262
Saini G, Leoni A, Franco S (1971) Solvent effects in radical copolymerization:1. Acrylamide Macromol Chem 144:235–244
Ryu JH, Han NK, Lee JS, Jeong YG (2019) Microstructure, thermal and mechanical properties of composite films based on carboxymethylated nanocellulose and polyacrylamide. Carbohyd Polym 211:84–90
Acknowledgements
We thank the National Natural Science Foundation of China (No. 51674117, 52173279), the Provincial Natural Science Foundation of China’s Hunan Province (No. 2020JJ4332), Scientific Research Fund of Hunan Provincial Education Department (No. 20A220, 20A215), the Key Laboratory of Hunan Province for Advanced Carbon-based Functional Materials, School of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, 414006, China, and Hunan Province (Xiangcai Construction Hunan Province, Xiangcai Construction).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, X., Liang, E., Zhou, F. et al. Acrylamide-ran-methyl methacrylate copolymers synthesized by copper(0)-catalyzed living radical polymerization. Iran Polym J 31, 983–990 (2022). https://doi.org/10.1007/s13726-022-01050-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-022-01050-y