Abstract
We report the findings of a 60-year-old female patient with metastatic breast cancer who presented with severe edema and neuralgia in the contralateral arm after receiving the third COVID-19 vaccine dose. The patient did not report any reaction to the first two doses of the BNT162b2 (Pfizer-BioNTech) vaccine. However, after a booster dose with the mRNA-1273 (Moderna) vaccine, the patient developed a high fever persisting for one week after the shot, and sequential severe swelling, inflammation, and pain in the contralateral arm lasting for three weeks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The third dose of the COVID-19 vaccine is highly effective in preventing infection, hospitalization, or death and is the standard of care worldwide. Previous reports [1] have shown that both allogeneic and heterologous booster vaccines have acceptable safety profiles and are immunogenic in adults who receive the primary COVID-19 vaccine. However, there are still limited data available to draw conclusions regarding the safety and efficacy of booster shots, especially for immunocompromised individuals such as cancer patients. To date, no case reports of local reactions away from the vaccination site have been reported, and the delayed local skin reaction "COVID arm," a side effect of the vaccine, has been reported only on the injection side. Here, we present the first clinical case of COVID arm developing at a site distant from the vaccination site in a patient who received a heterologous booster vaccine during systemic and local therapy for metastatic breast cancer.
Our case report suggests that cancer comorbidity and systemic or local therapy may affect the immune response to the COVID-19 vaccine and should be monitored closely.
Case report
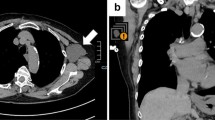
A 60-year-old woman was diagnosed with cT2N0M0 stage IIA breast cancer 12 years earlier and underwent a lumpectomy and sentinel node biopsy, followed by adjuvant chemotherapy and hormone therapy. Five years after surgery, she received a 54 Gray dose in 30 fractions of local radiation therapy and a second round of adjuvant chemotherapy for local recurrence in the right axilla and supraclavicular lymph nodes. Approximately 18 months after the local recurrence, she developed multiple lung metastases and subsequent metastases to her right breast. Therefore, she was newly treated with Fulvestrant and a cyclin dependent kinase 4/6 inhibitor one year prior to her first COVID-19 vaccination. During treatment, she received her first two inoculations of the BNT162b2 vaccine, with no adverse reactions. Four months after the first dose of BNT162b2, she received a 39 Gray dose in 13 fractions of local radiation therapy because of multiple cutaneous metastatic lesions in her left chest and left neck, and mild lymphadenopathy in her left arm. After radiation therapy, her left skin and arm symptoms resolved. Five months after radiation therapy, she received an additional dose of the mRNA-1273 vaccine in her right arm. The following day, she developed a high fever of 39 °C and fatigue. She then complained of severe swelling, redness, and pain in her left arm, the arm opposite the mRNA-1273 inoculation (Fig. 1). A computed tomography scan taken while her left arm was swollen showed no swelling of the left axillary lymph node nor abnormal findings on the left side. She was administered antibiotics for one week but the severe symptoms (redness, swelling, and pain) in the left upper extremity persisted for three weeks. During the course of treatment, her N-IgG antibodies were negative, and she had no previous infection with SARS-CoV-2. After booster administration, her S-IgG antibody concentration increased markedly to 8941.0 BAU/mL (Fig. 2).
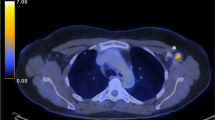
Photograph of swollen arm. Approximately three days after the third dose of mRNA-1273 vaccine, edema appeared in the left upper extremity. Heat and pain were observed in the same area. The image on the left shows the left upper extremity one week after vaccination. The image on the right shows the left upper extremity four weeks after vaccination
Graph of serum antibodies. Changes in serum antibodies before and after COVID-19 vaccination are shown. Blood antibodies that react specifically against the spike protein (S antigen) on the viral surface of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (SARS-CoV-2-S-IgG and SARS-CoV-2-S-IgM) and blood antibodies that react specifically against the nucleocapsid protein inside the virus (SARS-CoV-2-N-IgG) were tested with chemiluminescent enzyme immunoassay. The test kit was purchased from the Sysmex Corporation (Hyogo, Japan). The left vertical axis corresponds to S-IgG and the right vertical axis corresponds to N-IgG and S-IgM. SU/mL is the proprietary measurement unit of the Sysmex Corporation. The horizontal axis indicates the required number of days. There were four measurement points: before the patient’s first dose of COVID-19 vaccine, five weeks after her second dose of vaccine, seven months after her second dose of vaccine, and two weeks after the third dose of vaccine
Discussion
Here, we report a case of severe contralateral arm edema and neuralgia after a third dose of the COVID-19 vaccine, mRNA-1273 (Moderna), in the patient with metastatic breast cancer. With regard to the COVID-19 vaccine, delayed local skin reactions characterized by erythema, itching, pruritus, induration, and tenderness have been reported in many cases [2, 3]. However, there have been no reports of COVID arm development in the contralateral upper extremity after vaccination with the COVID-19 vaccine.
According to Català et al. [4], 32.3% of local skin reactions occur at the injection site and 67.7% exceed the injection site. In addition, there are six subtypes in order of frequency: (1) erythema or swollen plaques at the injection site (4 days or more after vaccination), (2) urticaria and/or angioedema on the torso or whole body (24 h or more after vaccination), (3) measle-like rash on the torso and extremities, (4) papulovesicular or pseudovesicular, (5) pityriasis rosea, and (6) purpuric rashes. These subtypes often develop on or after the fourth day of vaccination. In the present case, intense swelling, redness of the upper extremities, and severe pain were observed. Although this case was classified as subtype (1) based on the symptoms, it is inconsistent with previous reports because the disease occurred in the contralateral upper extremity. Other possible differential diagnoses included (1) cellulitis, (2) stasis dermatitis, (3) thrombophlebitis, (4) varicella-zoster virus, herpes simplex virus, and (5) radiation recall phenomenon (RRP). However, all were negative. The reasons are discussed below. (1) Cellulitis was ruled out because there was no increase in white blood counts (WBC) or inflammatory response during the course of the disease (Fig. 3). She had been taking antibiotics since the onset of her symptoms, but the fact that her symptoms did not disappear after a relatively long course of antibiotics also ruled it out. According to previous reports [3], the distinction between cellulitis and delayed cutaneous hypersensitivity reactions is also based on the median time of onset and the progressive reaction during the course of the disease. In this case, cellulitis was ruled out by the onset of symptoms approximately one week after vaccination and the absence of progressive reactions during the course of the disease. (2) Stasis dermatitis is usually seen on the lower extremities, while upper extremity stasis dermatitis is reported to be very rare, involving artificial AV fistulas [5]. This case was also negative because there were no varicose veins on the upper extremities and no venous hypertension. (3) Thrombophlebitis was ruled out because no obvious thrombus was noted on contrast-enhanced CT. (4) Reactivation of varicella-zoster virus and herpes simplex virus by COVID-19 vaccine is reported to account for about 10% of the vaccine reaction [4], but was not positively suspected based on skin findings. (5) RRP is a rare, delayed, acute inflammatory skin reaction that appears in a localized area consistent with the field of previous irradiation [6]. Although RRP has been reported with the COVID-19 vaccine [7], the extent of symptoms in this case was not consistent with the field of previous irradiation and was negative.
Serum antibody titers rose sharply after the third immunization, inducing a strong immune response compared to other patients undergoing cancer treatment [8]. These findings explained her symptoms as the COVID arm of the contralateral upper extremity was caused by mRNA-1273.
Based on initial studies to date, both allogeneic and heterologous COVID-19 booster regimens appear to be safe and immunogenic [1]. However, responses to each vaccine type vary among individuals. As seen in our case, the patient did not react to the first priming dose of the BNT162b2 vaccine but had severe systemic and local reactions to the mRNA-1273 vaccine. These reactions can be explained in two ways. First, there were differences in the type of vaccine. In general, there are reports that the mRNA-1273 vaccine causes slightly more adverse events than the BNT162b2 vaccine [9]. Next, we discussed the clinical course and treatment of the patient’s cancer. The patient had metastases on the left side of the upper body and was subsequently treated with local radiation therapy. According to a previous report [7], the spike protein sequence of the SARS-CoV-2 virus is genetically similar to human proteins through molecular mimicry and may induce immune responses, such as autoimmune diseases, after vaccination. In our case, severe swelling and redness followed by severe pain in the contralateral arm may have been a late adverse event associated with the systemic immune response. The left arm had undergone radiation therapy prior to the booster injection, and cancer cells remained throughout the entire upper left side, including the axilla. Therefore, the strongly induced immune response persisted only in this area, delaying the response. Both these cases could be attributed to the combination of systemic and local therapies, in addition to the vaccination affecting cancer patients. The response of patients with cancer to vaccination is often unknown because of the complexity of the therapeutic process. A previous report found no difference in seroprevalence among cancer patients compared to non-cancer healthcare workers [10]. However, the levels of IgG antibodies to nucleocapsid and spike proteins were significantly lower in patients with cancer, suggesting that COVID-19 itself or COVID-19 vaccination may differ between patients with cancer and the normal population. The patient in the present case did not receive immunosuppressive chemotherapy, which explains the high S-IgG levels. However, because of the unexpectedly high immune response, the patient experienced severe symptoms. This case suggests that concomitant cancer and systemic or local therapy may affect the immune response to the COVID-19 vaccine and that careful follow-up is necessary.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
15 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s13691-023-00600-2
References
Atmar RL, Lyke KE, Deming ME et al (2022) Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med 386(11):1046–1057. https://doi.org/10.1056/NEJMoa2116414
Johnston MS, Galan A, Watsky KL et al (2021) Delayed localized hypersensitivity reactions to the moderna COVID-19 vaccine: a case series. JAMA Dermatol 157(6):716–720. https://doi.org/10.1001/jamadermatol.2021.1214
Lindgren AL, Austin AH, Welsh KM (2021) COVID Arm: Delayed hypersensitivity reactions to SARS-CoV-2 vaccines misdiagnosed as cellulitis. J Prim Care Commun Health 12:21501327211024430. https://doi.org/10.1177/21501327211024431
Català A, Muñoz-Santos C, Galván-Casas C et al (2022) Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol 186(1):142–152. https://doi.org/10.1111/bjd.20639
Deguchi E, Imafuku S, Nakayama J (2010) Ulcerating stasis dermatitis of the forearm due to arteriovenous fistula: a case report and review of the published work. J Dermatol 37(6):550–553. https://doi.org/10.1111/j.1346-8138.2010.00857.x
Burris HA 3rd, Hurtig J (2010) Radiation recall with anticancer agents. Oncologist 15(11):1227–1237. https://doi.org/10.1634/theoncologist.2009-0090
Gambichler T, Boms S, Susok L et al (2022) Cutaneous findings following COVID-19 vaccination: review of world literature and own experience. J Eur Acad Dermatol Venereol 36(2):172–180. https://doi.org/10.1111/jdv.17744
Oosting SF, van der Veldt AAM, Fehrmann RSN et al (2022) Immunogenicity after second and third mRNA-1273 vaccination doses in patients receiving chemotherapy, immunotherapy, or both for solid tumours. Lancet Oncol 23(7):833–835. https://doi.org/10.1016/s1470-2045(22)00203-0
Beatty AL, Peyser ND, Butcher XE et al (2021) Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open 4(12):e2140364. https://doi.org/10.1001/jamanetworkopen.2021.40364
Yazaki S, Yoshida T, Kojima Y et al (2021) Difference in SARS-CoV-2 antibody status between patients with cancer and health care workers during the COVID-19 pandemic in Japan. JAMA Oncol 7(8):1141–1148. https://doi.org/10.1001/jamaoncol.2021.2159
Acknowledgements
We thank Dr. Yagishita for his cooperation in transporting plasma specimens.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No funding was provided for this study.
Ethical approval
The measurement of the serum antibodies was approved by the ethics review board of the National Cancer Center Central Hospital (No. 2014092) and was performed in full compliance with the Declaration of Helsinki.
Informed consent
Informed consent was obtained from the participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original article has been revised due to update in Figure 3.
About this article
Cite this article
Sanomachi, T., Sumiyoshi Okuma, H. & Yonemori, K. COVID arm that appeared in the contralateral upper extremity after mRNA-1273 booster inoculation. Int Canc Conf J 12, 216–219 (2023). https://doi.org/10.1007/s13691-023-00598-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13691-023-00598-7