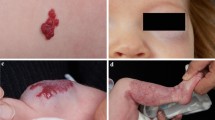
Abstract
Infantile hemangiomas (IHs) are the most common vascular tumors of infancy. Since it was discovered several years ago, propranolol hydrochloride has become a very popular and successful treatment for complicated and aesthetically disfiguring IHs. The recent literature on propranolol is reviewed, including a summary of larger studies, discussion of adverse effects, and the use of propranolol in complicated IHs, such as ulcerated IHs, airway, and hepatic IHs.
Similar content being viewed by others
Introduction
Infantile hemangiomas (IHs) are the most common vascular tumors of infancy affecting approximately 4 % of Caucasian infants [1]. IHs generally are not present at birth and subsequently proliferate with the most rapid growth period, particularly of superficial IHs occurring between 1 and 2 months of age [2•]. The majority of IH growth is completed by 5 months of age [3]. The rapid growth during early infancy is followed by slowed growth and gradual involution. Although many IHs do not require treatment, in certain anatomic areas IHs may cause disfigurement or threaten vital functions (such as visual, oral, or airway function). IHs may become complicated with painful ulceration or, rarely, bleeding.
Propranolol hydrochloride is a nonselective B-blocker that was serendipitously discovered to inhibit growth of IHs in 2008 when children with IHs were treated with propranolol for cardiac indications [4•]. Since that time, there have been an outpouring of reports describing the use of propranolol, including numerous case series and a small, randomized, controlled trial supporting the safety and efficacy of propranolol [5•]. Other beta blockers, such as nadalol, acebutolol, atenolol, and topical timolol, have been reported to be beneficial; however, the majority of reports are of propranolol. Although there are no FDA-approved treatments for infantile hemangiomas, the advent of propranolol is a major advance for the field of Pediatric Dermatology. The advantages of propranolol are that it is has a rapid onset resulting in clinical improvement, appears to have less serious side effects than systemic corticosteroids, can be used for IHs beyond the proliferative phase, and is inexpensive. A large, international, randomized, controlled trial is underway. Propranolol has become the first-line treatment for IHs, eclipsing systemic corticosteroids as the preferred treatment for IHs. This review article summarizes the recent literature for propranolol as a treatment for infantile hemangiomas.
Discussion
Pharmacology
Propranolol hydrochloride is used to treat hypertension, migraine headaches, anxiety, and tremor. In infants, it has primarily been used for cardiac indications and neonatal hyperthyroidism. Propranolol has not been FDA approved for any pediatric indication; however, in other countries propranolol is licensed for pediatric use in migraine prophylaxis, arrhythmias, thyrotoxicosis, and tetralogy of Fallot [6]. Propranolol acts on beta-1 (cardiac) and beta-2 (bronchial, vascular smooth muscle) receptors. Beta-1 blockade produces decreased heart rate and decreased myocardial contractility during periods of high sympathetic activity, resulting in decreased cardiac output. Blockade of beta receptors in cardiac conduction tissue results in slowing AV conduction and suppression of automaticity. Beta-2 blockade is responsible for many of the adverse effects of propranolol, including bronchospasm, hypoglycemia, and peripheral vasoconstriction. Propranolol has been well studied in adults and has a therapeutic half life of 3–6 hours [7].
Mechanism of Action
The mechanism of propranolol acting on infantile hemangiomas is being investigated. Beta adrenergic receptors are present on endothelial cells. Mechanisms, such as vasoconstriction, endothelial cell apoptosis, and decreased angiogenesis, have been proposed to explain how propranolol affects IHs.
Beta blockers inhibit vasodilation, which is caused by adrenaline interacting with beta receptors, resulting in nitrous oxide synthesis and release. Vasoconstriction leads to immediate changes in the IH due to decreased blood flow from the capillaries feeding the IH and can be observed as color lightening and softening within the first 3 days of initiating treatment [8•].
Angiogenic growth factors are important in endothelial cell proliferation. Beta blockers are proposed to downregulate angiogenic growth factors, such as VEGFA, matrix metalloproteinases (MMP-2 and MMP-9), and IL-6.[9] Chim et al. treated proliferating and regressing IH endothelial cells with propranolol and found that propranolol induces a cytotoxic effect on IH endothelial cells through HIF 1alpha mediated inhibition of VEGF-A [10].
Propranolol Studies
All studies to date have indicated that propranolol is effective for treating infantile hemangiomas [5•, 11•, 12–17]. Most studies evaluating propranolol for infantile hemangiomas have involved small patient numbers with dosages of 1–3 mg/kg/day divided bid to tid (Table 1). In young infants, less than 1–2 months of age, some physicians initiate therapy during a brief hospitalization. In older patients, propranolol is usually administered on an outpatient basis. Some centers gradually titrate the dosage over a 1- to 2-week period, versus others who initiate the full dose of oral propranolol at 2 mg/kg/day. Duration of treatment ranges from 3 to 9 months. Propranolol has been used to treat children of different ages with IHs in the proliferative phase, as well as stable IHs. Most studies for propranolol established a protocol, including pretreatment cardiac evaluation (sometimes with EKG and echocardiogram), followed by monitoring of vital signs, and sometimes glucose levels or ultrasound measurements of the IH. Most studies have excluded patients with PHACE syndrome and arterial abnormalities by performing MRI/MRA on infants with large facial IHs. Some groups admit patients to the hospital for monitoring hemodynamic parameters after the first dose, whereas in clinical dermatology practice propranolol is usually initiated on an outpatient basis. A consensus conference recently convened to standardize the approach to propranolol use in infantile hemangioma. These results have not yet been published. Discontinuing propranolol is recommended by tapering the dosage over a 2-week period to prevent rebound tachycardia. Rebound IH growth tends to occur in children younger than 12 months [5•]. A recent meta-analysis of greater than 1,000 patients treated with propranolol for IH with a mean dose of 2.1 mg/kg/day showed a high rate of efficacy (average 97 %) and very low rate of serious adverse events. The most common adverse events were sleep disturbances and acrocyanosis. Serious adverse events were rare, with reports of symptomatic hypotension in five patients, symptomatic bradycardia in one patient, and hypoglycemia in four patients, one of which presented with hypoglycemic seizures [18]. Larger, prospective, randomized, controlled trials are expected to provide more information about the optimal dosage, efficacy, and safety profile of propranolol.
Comparing Propranolol and Systemic Corticosteroids
Propranolol seems to be superior to systemic corticosteroids. This has been assessed in two, small, comparative trials. A retrospective case control study, matched 12 pairs of infants who received propranolol with those that had received systemic corticosteroids, by IH size, location, and age. They found that at 6 months of treatment, all of the children in the propranolol group showed good-to-excellent response, whereas nine children in the corticosteroid group showed slight-to-moderate response. The response in the propranolol group was faster and showed greater clinical improvement [19].
A retrospective comparison of 68 children who received propranolol for their IHs with 42 patients that received corticosteroids for their IHs, found that 81 % of the propranolol group achieved 75 % improvement in their IH compared with 29 % of the corticosteroid group with 75 % improvement. Additionally, the cost of propranolol was less, and there were fewer complications in the propranolol group [20].
Ulcerated IHs
Propranolol is a promising treatment for ulcerated IHs. The most common complication of infantile hemangiomas is ulceration, in up to 15–25 % of IHs in a tertiary care setting [21, 22]. Treatment of ulceration includes general wound care measures with debridement of crust via saline soaks, application of topical antibiotics, pain medications, and wound dressings. Medical and surgical treatments employed for ulcerated IHs include becaplermin gel, pulsed dye laser therapy, and surgical excision of the ulcerated IH. There are reports of propranolol being effective in the healing process of ulcerated IHs. Propranolol was effective in treating 33 infants with problematic ulcerated hemangiomas in a retrospective review [23]. The mean time to healing was 4.3 weeks, and head and neck location of the IH correlated with faster healing. Children also were reported to have significantly reduced pain within days of starting propranolol treatment. In a case-control study of 20 infants with ulcerated IHs treated with propranolol matched to historical controls, the propranolol group healed faster than the control group 8.7 vs 22.4 weeks. Starting propranolol at an earlier stage of disease tended to result in shorter ulceration duration [24]. In six infants with complicated ulcerated IHs treated with propranolol, the ulcerations healed within 2–6 weeks of starting propranolol [25]. These reports all used a dosage of 2–2.5 mg/kg/day divided tid. Bagazgoitia et al. included eight ulcerated IHs in their study where they used 2 mg/kg/day divided bid; all ulcerations healed within 2 weeks of propranolol [11•]. In contrast, Manunza et al. reported that two of their patients with deep ulcerations deteriorated despite treatment with propranolol [14]. Although larger confirmatory studies are likely required, some authors are recommending propranolol as a first-line treatment for ulcerated IH. It may be helpful for the clinician to identify and treat high-risk IHs (such as a large superficial component, located on mucosal surfaces, the perineum, or in areas of high friction, and with early white or greyish surface discoloration) to try to avoid ulceration.
Hepatic IH and Propranolol
The liver is the most common extracutaneous site of IH involvement. Hepatic infantile hemangiomas can be classified as focal, multifocal, and diffuse. Similar to cutaneous IHs, liver IHs are usually small, localized, and do not require treatment. Certain liver IHs can result in life-threatening complications, such as high-output cardiac failure or liver enlargement leading to hypothyroidism. Hypothyroidism is due to deactivation of thyroxine by type 3 iodothyronine deiodinase produced by hemangioma tissue where the IH replaces most of the liver or in very large IHs [26]. The presence of numerous cutaneous IH can be an important clue to the diagnosis of hepatic IH, and abdominal ultrasound is recommended if five or more cutaneous IHs are identified. Diffuse hepatic infantile hemangiomas (a rare subtype) have been treated successfully by using propranolol in combination with other medical therapies, such as systemic corticosteroids and vincristine [27]. Mhanna et al. successfully treated three infants with multifocal and diffuse intrahepatic hemangiomas with propranolol at a dosage range of 1.5–2 mg/kg/day [28]. Mazereeuw-Hautier described eight patients with hepatic IH, three of whom were experiencing heart failure who were treated with propranolol. All of the reported patients experienced clinical or radiographic improvement, or both, including resolution of the associated hypothyroidism in three patients with diffuse hepatic lesions [29]. The use of propranolol to treat symptomatic hepatic IH requires further study to determine optimal dosage and use as a monotherapy or to be used in conjunction with steroids and vincristine.
PHACE Syndrome and Propranolol
PHACE syndrome (posterior fossa abnormalities, hemangioma, arterial/aortic anomalies, cardiac anomalies, eye abnormalities, and sternal/supraumbilical raphe) is an uncommon neurocutaneous disorder where infants present with large (>5 cm), facial hemangiomas.
Propranolol use in PHACE syndrome remains controversial, because there is concern that propranolol could increase the risk for stroke in this patient population. A recent poster described propranolol use in 32 patients with PHACE syndrome with cervical and intracranial artery anomalies. These children were treated with an average dose of 1.8 mg/kg/day divided tid or bid. One of 32 patients developed a change in neurologic status. Recommendations were to use the lowest possible dosage of propranolol, slow titration, tid dosing to minimize abrupt changes in BP, and close patient follow-up [30]. PHACE syndrome is not an absolute contraindication to propranolol use, but must be considered and monitored carefully, particularly if the child has aberrant cerebrovasculature.
Airway IH and Propranolol
Airway hemangiomas can be life-threatening. Children with IHs in the beard or mandibular distribution (facial segment S3) are at risk of developing airway IHs. Children with PHACE syndrome have been recently reported to have up to 52 % risk of airway hemangiomas [31]. Rarely, IHs in other locations can be associated with airway IHs, and these children present with stridor, hoarse cry, or difficulty breathing in the first 4–12 weeks of life. Traditional treatment of airway IHs is with systemic corticosteroids, laser surgery performed by ENT, and rarely tracheostomy. There are case reports and case series of propranolol successfully treating airway IHs [11•, 14, 15, 32]. A meta-analysis of 36 infants with airway IHs concluded that propranolol should be recommended as a first-line treatment in infantile airway hemangiomas. A full cardiovascular and respiratory evaluation is recommended before initiation of propranolol in these children, and inpatient monitoring is likely required for children with respiratory symptoms [33].
Propranolol Used to Treat IH Beyond the Proliferative Phase
Propranolol is effective to treat IHs beyond the proliferative phase. A retrospective multicenter study of 49 patients confirmed that propranolol is effective to shrink IH that are beyond the proliferative phase, including effectiveness in a 10-year-old child. The authors suggest using propranolol in older infants before surgical intervention, because it may minimize the need for plastic surgery or result in a smaller excision [34•]. Propranolol was as effective in children older than age 6 months as it was in children younger than age 6 months in the largest, retrospective, multicenter, case series to date [11•]. Propranolol was effective in children older than age 6 months who were treated in a randomized, controlled trial [5•].
Safety/Adverse Effects
Since the advent of systemic propranolol therapy for IH, pediatric dermatologists and those caring for infants with IH have become familiar with the side effects and contraindications of beta adrenergic blocking medications (Table 2). The adverse effects of propranolol are well known and include bradycardia, hypotension, bronchospasm, hypoglycemia, sleep disturbance, nightmares, and acrocyanosis. Gastroesophageal reflux, nausea, vomiting, diarrhea, somnolence, hyperkalemia, tumor lysis syndrome, psoriatic drug rash, respiratory syncytial virus exacerbation, and dental caries have been reported [35–38]. Propranolol is contraindicated in patients with asthma, heart block, and sinus bradycardia, and it is recommended not to be used during episodes of bronchiolitis. Caution is advised in patients with PHACE syndrome and cervical or intracranial arterial abnormalities. The most serious side effect of propranolol is hypoglycemia. Propranolol is thought to cause hypoglycemia by inhibiting glycogenolysis, glyconeogenesis, and lipolysis. Children have lower glycogen stores and higher glucose consumption rates when fasting and, therefore, are more susceptible to hypoglycemia than adults [39•]. It is suggested to administer propranolol with feeds to reduce the risk of hypoglycemia. Therapy should be temporarily discontinued in the event of intercurrent illness, such as vomiting, diarrhea, decreased oral intake, somnolence, or bronchiolitis. An uncontrolled case series showed a high rate (64 %) of mostly minor side effects in 18 of 28 infants treated with propranolol for IHs [40]. Controlled studies will be helpful to further delineate adverse events attributable to propranolol in this patient population.
Rebound Growth of IH
Pediatric dermatologists have noticed that when propranolol treatment is ceased, there may be rebound growth of the IH or changes, such as increased redness and elevation of the IH.
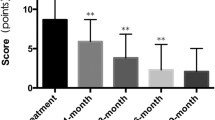
One case series has specifically looked at this issue and found that 5 of 24 patients (19 %) had rebound IH growth within 0 to 6 months of stopping oral propranolol. Early cessation of treatment or a prolonged IH proliferative phase may be the reason for rebound growth, but the exact mechanism remains unknown. It was mainly the deep component that recurred [41]. The patients in this study also mainly had deep segmental IH, which have been known to have a prolonged proliferative phase [42]. It is important to follow-up with patients after discontinuing propranolol (for up to 6 months) to assess for potential rebound and intervene as necessary, especially if the children are younger than 1 year of age. In my experience, if you have access to an experienced ultrasonographer, performing an ultrasound before stopping propranolol in patients with deep IHs may be helpful to assess a residual deep component with high blood flow, which is likely to rebound.
In a randomized, controlled trial, increased redness and increase in volume was noted after discontinuing propranolol. This was more common in children younger than 1 year of age [5•].
Topical Beta Blockers
Timolol is a topical nonselective beta-blocker that is the most commonly prescribed therapy for childhood glaucoma. Timolol in its ophthalmic administration is contraindicated in patients with asthma, sinus bradycardia, atrioventricular block, or overt cardiac failure. Timolol has showed therapeutic promise for IH in a few case reports and small pilot studies. For superficial (thin) hemangiomas, at least, topical timolol may be effective. The largest study to date provides encouraging results. It describes the use of timolol maleate 0.5 % or 0.1 % gel forming solution for small, superficial IH retrospectively in a multicenter study involving 73 children. The majority (85 %) were treated with the 0.5 % solution twice daily without occlusion for approximately 3–6 months. All children improved except one, and one child had sleep disturbances. No rebound growth occurred after 3–6 months of follow-up [43]. The mechanism of timolol is presumably similar to that of propranolol. Given intravenously, timolol has been shown to be eight times as potent as propranolol. Topical timolol gel is thought to be less bioavailable for systemic absorption than topical timolol solution. A recent commentary cautions those prescribing topical timolol to be aware of side effects and systemic absorption, which may be even more pronounced in certain locations, such as mucosal surface and thinner skin sites, such as periocular, oral, perineal, perianal, and ulcerated IH. Using a topical medication also may delay use of a systemic medication in cases where systemic agents would be more appropriate. Recommendations include telling parents to use an exact number of drops, such as 1–2 drops per application to help avoid overdose, as well as monitoring for fussiness, poor circulation, and hypothermia [44]. Because systemic absorption likely occurs with topical timolol, patient’s vital signs should be monitored. Two small studies have assessed topical 1 % propranolol applied bid or tid to superficial IHs and found that it was effective [45, 46]. Randomized, controlled studies of the safety and efficacy of topical beta-blockers for IH are in process. Future studies should address the optimal dosage concentration, duration of treatment, systemic absorption of topical timolol and topical propranolol, and mode of application. Until we know more, systemic therapy should probably remain first-line therapy for time-sensitive problematic hemangiomas, such as those that threaten vision.
Conclusions
Treating IHs with oral propranolol hydrochloride 2 mg/kg/day divided bid to tid until the end of the proliferative phase appears to be very effective, and the evidence to date supports its use as a first-line option for potentially disfiguring or complicated IHs. Larger dose-finding studies are required to confirm the optimal dosage of propranolol. Due to the heterogeneous nature of IHs, duration of treatment with propranolol will likely need to be individualized depending on the child’s type of IH and response to treatment.
Guidelines for initiating treatment with propranolol and therapeutic monitoring vary widely between institutions. Until consensus practice guidelines for propranolol and IHs are published, it is recommended that dermatologists communicate with their local pediatric cardiologists to establish their practice parameters. There may be unknown side effects of propranolol used for the indication of infantile hemangiomas and regular follow-up visits are advised. Although there may be pressure from parents to treat uncomplicated, nondisfiguring IHs, propranolol is not without side effects, and it is important to weigh the risks and benefits of treatment, as well as to reassure parents about the natural history of IHs and eventual involution. Evidence from larger, controlled trials should be helpful to answer outstanding questions about propranolol, particularly safety profile.
Abbreviations
- IH:
-
Infantile hemangioma
References
Papers of particular interest have been highlighted as: • Of importance
Frieden IJ, Haggstrom AN, Drolet BA, et al. Infantile hemangiomas: current knowledge, future directions. Proceedings of a research workshop on infantile hemangiomas. April 7–9, 2005. Bethesda, Maryland. Pediatr Dermatol. 2005;22(5):383–406.
• Tollefson MM, Frieden IJ. Early growth of infantile hemangiomas: what parents’ photographs tell us. Pediatrics 2012 Jul 23. (Epub ahead of print) This study reinforces that early referral to a pediatric dermatologist is key in the management of high-risk IHs, and 4 weeks may be an ideal age for these infants to be seen for first consultation.
Chang LC, Haggstrom AN, Drolet BA, Hemangioma Investigator Group, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122(2):360–7.
• Leaute-Labreze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358(24):2649–51. Discovery that propranolol is effective in treating IH.
• Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128(2):e259–66. First RCT, albeit it small, to demonstrate safety and efficacy of propranolol compared with placebo.
Starkey E, Shahidullah H. Propranolol for infantile haemangiomas: a review. Arch Dis Child. 2011;96:860–93.
Lalonde RL, Pieper JA, Straka RJ, et al. Propranolol pharmacokinetics and pharmacodynamics after single doses and at steady-state. Eur J Clin Pharmacol. 1987;32:315–8.
• Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol. 2010;168(2):269–74. Good review of possible mechanisms for propranolol.
Greenberger S, Bischoff J. Infantile hemangioma-mechanism(s) of drug action on a vascular tumor. Cold Spring Harb Perspect Med. 2011;1(1):a006460.
Chim H, Armijo BS, Miller E, et al. Propranolol induces regression of hemangioma cells through HIF-1α mediated inhibition of VEGF-A. Ann Surg. 2012 May 10 (epub ahead of print)
• Bagazgoitia L, Torrelo A, Lopez Gutierrez JC, et al. Propranolol for infantile hemangiomas. Pediatr Dermatol. 2011;28(2):108–14. This is the largest case series published to date.
Schupp CJ, Kleber JB, Gunther P, et al. Propranolol therapy in 55 infants with infantile hemangioma: dosage, duration, adverse effects, and outcome. Pediatr Dermatol. 2011;28(6):640–4.
Bertrand J, Sammour R, McCuaig C, et al. Propranolol in the treatment of problematic infantile hemangiomas a review of 35 consecutive patients at a vascular anomalies clinic. J C Cutan Med Surg. 2012;16(2):115–21.
Manunza F, Syed S, Laguda B, et al. Propranolol for complicated infantile haemangiomas: a case series of 30 infants. Br J Dermatol. 2010;163(2):466–8.
Sans V, de la Roque ED, Berge J, et al. Propranolol for severe infantile hemangiomas: follow up report. Pediatrics. 2009;124(3).
Holmes WJ, Mishra A, Gorst C, et al. Propranolol as first line treatment for rapidly proliferating infantile haemangiomas. J Plast Reconstr Aesthet Surg. 2011;64(4):445–51.
Buckmiller LM, Munson PD, Dyamenahalli U, et al. Propranolol for infantile hemangiomas: early experience at a tertiary vascular anomalies center. Laryngoscope. 2010;120(4):676–81.
Marqueeling AL, Frieden IJ. A meta-analysis of propranolol for infantile hemangiomas. Poster. Presented at the Society for Pediatric Dermatology 38th annual meeting, Monterey, CA, July 11–14, 2012.
Bertrand J, McCuaig C, Dubois J, et al. Propranolol versus Prednisone in the treatment of infantile hemangiomas: a retrospective comparative study. Pediatr Dermatol. 2011;28(6):649–54.
Price CJ, Lattouf C, Baum B, et al. Propranolol vs corticosteroids for infantile hemangiomas: a multicenter retrospective analysis. Arch Dermatol. 2011;147(12):1371–6.
Hermans DJ, Boezeman JB, Van de Kerkhof PC, et al. Differences between ulcerated and non-ulcerated hemangiomas, a retrospective study of 465 cases. Eur J Dermatol. 2009;19:152–6.
Chamlin SL, Haggstrom AN, Drolet BA, et al. Multicenter prospective study of ulcerated hemangiomas. J Pediatr. 2007;151:684–9.
Saint-Jean M, Leaute-Labreze C, Mazereeuw-Hautier J, et al. Propranolol for treatment of ulcerated infantile hemangiomas. J Am Acad Dermatol. 2011;5:827–32.
Hermans DJ, van Beynum IM, Schultze Kool LJ, et al. Propranolol, a very promising treatment for ulceration in infantile hemangiomas: a study of 20 cases with matched historical controls. J Am Acad Dermatol. 2011;64(5):833–8.
Kim LHC, Hogeling M, Wargon O, et al. Propranolol: useful therapeutic agent for the treatment of ulcerated infantile hemangiomas. J Ped Surg. 2011;46:759–63.
Huang SA, Tu HM, Harney JW, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000;343:185–9.
Yeh I, Bruckner AL, Sanchez R, et al. Diffuse infantile hepatic hemangiomas: a report of four cases successfully managed with medical therapy. Pediatr Dermatol. 2011;28(3):267–75.
Mhanna A, Franklin WH, Mancini AJ. Hepatic Infantile Hemangiomas treated with oral propranolol—a case series. Pediatr Dermatol. 2011;28(1):39–45.
Mazereeuw-Hautier J, Hoeger PH, Benlahrech S, et al. Efficacy of propranolol in hepatic infantile hemangiomas with diffuse neonatal hemangiomatosis. J Pediatr. 2010;157:340–2.
Metry DW. Propranolol use PHACE syndrome with cervical and intracranial arterial anomalies: collective experience in 32 infants. Poster. Presented at the Society for Pediatric Dermatology 38th annual meeting, Monterey, CA. July 11–14, 2012.
Durr ML, Meyer AK, Huoh KH, et al. Airway hemangiomas in PHACE syndrome. Laryngoscope. 2012 Aug 2. doi:10.1002/lary.23475 (Epub ahead of print).
Rosbe KW, Suh KY, Meyer AK, et al. Propranolol in the management of airway infantile hemangiomas. Arch Otolaryngol Head Neck Surg. 2010;136(7):658–65.
Peridis S, Pilgrim G, Athanasopoulos I, et al. A meta-analysis on the effectiveness of propranolol for the treatment of infantile airway haemangiomas. Int J Pediatr Otorhinolaryngol. 2011;75(4):455–60.
• Zvulunov A, McCuaig C, Frieden IJ, et al. Oral propranolol therapy for infantile hemangiomas beyond the proliferative phase: a multicenter retrospective study. Pediatr Dermatol. 2011;28(2):94–8. This study showed that propranolol is effective, even for fully formed IHs.
Lawley LP, Siegfried E, Todd JL. Propranolol treatment for hemangioma of infancy: risks and recommendations. Pediatr Dermatol. 2009;26(5):610–4.
Pavlakovic H, Kietz S, Lauerer P, et al. Hyperkalemia complicating propranolol treatment of an infantile hemangioma. Pediatrics. 2010;126(6).
Abbot J, Paruleker M, Shahidullah H, et al. Diarrhea associated with propranolol treatment for hemangioma of infancy. Pediatr Dermatol. 2010;27(5):558.
Giron-Vallejo O, Lopez-Gutierrez JC, Fernandez-Pineda I, et al. Dental caries as a side effect of infantile hemangioma treatment with propranolol solution. Pediatr Dermatol. 2010;27(6):672–3.
• Holland KE, Frieden IJ, Frommelt PC, et al. Hypoglycemia in children taking propranolol for the treatment of infantile hemangioma. Arch Dermatol. 2010;146(7):775–8. A good review of hypoglycemia and propranolol.
de Graaf M, Breur JM, Raphael MF, et al. Adverse events of propranolol when used in the treatment of hemangiomas: a case series of 28 infants. J Am Acad Dermatol. 2011;65(2):320–7.
Bagazgoitia L, Hernandez-Martin A, Torrelo A. Recurrence of infantile hemangiomas treated with propranolol. Pediatr Dermatol. 2011;28(6):658–62.
Brandling-Bennett HA, Metry DW, Baselga E, et al. Infantile hemangiomas with unusually prolonged growth phase: a case series. Arch Dermatol. 2008;144(12):1632–7.
Chakkittakandiyil A, Phillips R, Frieden IJ, et al. Timolol maleate 0.5% or 0.1% gel forming solution for infantile hemangiomas: a retrospective, multicenter, cohort study. Pediatr Dermatol. 2012;29(1):28–31.
McMahon P, Oza V, Frieden IJ. Topical timolol for infantile hemangiomas: putting a note of caution in “cautiously optimistic. Pediatr Dermatol. 2012;29(1):127–30.
Kunzi-Rapp K. Topical propranolol therapy for infantile hemangiomas. Pediatr Dermatol. 2012;29(2):154–9.
Xu G, Lv R, Zhao R, et al. Topical propranolol for treatment of superficial infantile hemangiomas. J Am Acad Dermatol. 2012 Apr 17. (epub ahead of print)
Disclosure
The author has reported no potential conflicts of interest relevant to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hogeling, M. Propranolol for Infantile Hemangiomas: A Review. Curr Derm Rep 1, 179–185 (2012). https://doi.org/10.1007/s13671-012-0026-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13671-012-0026-6