Abstract
Current-activated tip-based sintering (CATS) is a new process that imposes local current-activated sintering conditions to consolidate selected areas of a powder compact/bed through the controlled application of a contacting tip electrode. The process has the ability of achieving very high sintering rates and obtaining complex-sintered geometries through the controlled precision motion of the electrically conductive tip. In this study, the high current densities afforded by CATS are utilized to locally activate a macroscopic combustion synthesis type reaction in compacts of reactive mixtures of nickel and aluminum to rapidly form nickel aluminides. The effect of current intensity on the ignition time, microstructure, homogeneity, and properties of the combustion synthesized products is discussed in this article. It was found that ultra-rapid formation of aluminum-rich intermetallics precedes and contributes to the major ignition event. Moreover, time to ignition was found to decrease with an increase in current intensity, also leading to less homogenous microstructures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spark plasma sintering (SPS) has been at the center of recent scientific attention as a rapid powder consolidation process [1–6]. Advantages of the process over conventional sintering include rapid heating rates, shorter sintering times, and lower sintering temperatures. Recently, a novel process “current-activated tip-based sintering (CATS)” [7] was developed by three of the authors, wherein electric current is locally applied through an electrically conducting precision controlled tip that is either placed or allowed to travel over a powder bed/compact to locally sinter small targeted regions. With this process, one-, two-, and three-dimensional microscale (and potentially nanoscale) sintered features can be formed very rapidly. The process has so far been limited to the production of high-density, locally sintered regions from non-reactive powders [7, 8]. The CATS of reactive mixtures of powders, however, can present new possibilities for either locally imposing a microscale combustion synthesis process or for activating a macroscale combustion synthesis process. In the conventional combustion synthesis (thermal explosion mode) of Ni–Al materials [9], compacts of nickel and aluminum powder mixtures are heated to an ignition temperature at which the reactant powders are converted to the intended intermetallic product. The reaction is exothermic in nature, which gives rise to considerable self-heating of the compact, thus instantaneously raising the compact temperature by several hundred degrees. Combustion synthesis within the self-propagating high temperature synthesis (SHS) mode of ignition has also been applied to the synthesis of nickel aluminides [9]. In this case, the powder compact is ignited (using radiative or laser heating) at one end giving rise to a local reaction that spreads in the form of a wave throughout the compact, consuming reactants and precipitating products in its wake. Recent preliminary experiments by the authors on reactive current-activated tip-based sintering (R-CATS) have indicated that very low green densities with an accompanied low thermal and electrical conductivity can play an important role in localizing/limiting the reactions to regions close to the tip [10]. However, if reactions were intentionally allowed to propagate beyond the local ignition point, R-CATS can be a viable inexpensive process for “current-activated” combustion synthesis of macroscale products. As such, this article explores for the first time, the R-CATS of macroscale reactively produced materials. The effect of current intensity on the ignition time, developed microstructure, homogeneity, and properties is discussed for reactive compacts of nickel and aluminum powder mixtures.
Experimental Procedure
Precurser materials used in the study were nickel (Ni) powder (3–7 μm, INCO 123) and aluminum (Al) powder (325 mesh). Powders were rotator mixed for 30 min at 70 rpm in the composition of Ni3Al (86.7 wt% Ni with balance aluminum). Figure 1 shows scanning electron micrographs of the nickel and aluminum powders. The relative sizes and morphologies of the powders used are shown, which are ligamental in the case of aluminum (Fig. 1a) and spherical/rounded in the case of nickel with pronounced evidence of agglomeration for 3- to 7-μm nickel (Fig. 1b). The Ni/Al powder mixture was first placed inside the die, and tapped multiple times for leveling then compacted with a pressure of 369 MPa to produce green compacts of ~75% theoretical green density. While still inside the die, a very thin layer of nickel powder was likewise added and the whole arrangement repressed at a lower pressure of 246 MPa. This resulted in green compact of ~14 mm diameter and 2.5 mm thick, with a ~300-μm-thick top layer comprised only of nickel (i.e., aluminum free). The exclusion of aluminum in the top layer was to avoid severe tip/specimen reactions brought upon by contact with molten aluminum.
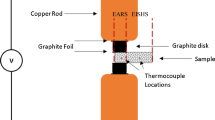
All compacts were vacuum degassed at 120 °C for 1 h prior to reactive processing. The R-CATS setup used is shown in Fig. 2. A tungsten tip with a flat contacting surface 1 mm in diameter was used to deliver a continuous DC electric current centrally to the top surface of the green compact, and promote local ignition. Three current intensities were investigated (100, 200, and 300 A), and following any given reaction, the power was immediately switched off.
The powder shape, morphology, and post processing microstructure were analyzed using a field emission scanning electron microscope (FESEM). For compositional and phase analysis, energy dispersive X-ray spectroscopy (EDXS) and X-ray diffraction (XRD) were used. Microhardness was evaluated using a Wilson microhardness tester with a load of 500 g. At least seven indents were made for each specimen and an average and standard deviation was calculated and presented. Porosity measurements were conducted using image analysis; for any given location, at least three images were used in the analysis.
Results and Discussion
Figure 3 shows the tip–specimen configuration and reaction when 200 A current was applied. The initial radial glow on the surface of the sample is believed to be caused by reflection of the light from the hot tip onto the specimen, and not a reaction wave propagation. Similar observations were noted when non-reacting powder compacts were used in other studies. However, what is clear is the formation of a fine crack (due to local rapid rise in temperature) at around 16.45 s, which is believed to correspond to the onset of an SHS type reaction, with very rapid observed wave propagation that engulfs the whole specimen within ~0.5 s (Fig. 3h).
To reveal the local microstructural evolution leading up to ignition, specimens where exposed to 200 A current for 5, 10, and 15 s (i.e., just before ignition occurs). Microstructural characterization of the material region beneath the tip reveals the formation of some aluminum-rich intermetallic phases even after 5 s of current exposure. The size of the Al-rich intermetallic region was found to increase as the current exposure time increased from 5 to 15 s.
Figure 4 shows the microstructure of the specimen cross-section beneath the tip after 15 s of current exposure. EDXS analysis shows that just below the initial aluminum-free nickel layer, Al3Ni and Al3Ni2 (also shown in Fig. 4) have formed in the Ni/Al region beneath the tip (similar microstructures were also observed for the 300 A samples after exposure times of 3 s), and no aluminum powder was present in this region. In conventional combustion synthesis of nickel aluminides, such Al-rich intermetallic (Al3Ni and Al3Ni2) phase formation has been known to occur when heating Ni + Al green compacts at very low heating rates (for example less than 10 °C/min) to the ignition temperature (typically 640 °C for this material system) [9]. This gives more time prior to ignition for solid-state interdiffusion to occur between aluminum and nickel where aluminum can become significantly consumed at the lower heating rates, leaving less aluminum for the major intended reaction. In this case, however, the very rapid heating rates imposed by the local current exposure (taking only ~5 and ~16.5 s to reach the ignition temperature under current intensities of 300 and 200 A, respectively) points to another more likely electric current activation effect that increases the rate of solid-state interdiffusion between nickel and aluminum. Previous study on Ni/Ti foil diffusion couples have indeed shown that electric current can have a major influence on the diffusion rate and consequently the rate of intermetallic phase growth which can be more than an order of magnitude higher than for diffusion couples carried out at the same temperature but without current exposure [11]. Hence our rapid aluminum-rich intermetallic phase formation in such a short time is believed to be due to the high current densities locally imposed via CATS.
It is interesting to see from Fig. 4 that at the bottom surface, a similar region was observed, where aluminum-rich intermetallics have formed prior to ignition. The formation of these Al-rich intermetallic phases is exothermic in nature, which gives rise to local self-heating that supplements and modifies the Joule heating imposed by the tip. It is therefore believed that the SHS reaction propagates from one or both of these regions. Figure 4 also shows that even at 15 s of current exposure the interior of the specimen is still a mixture of nickel and aluminum powder with no observed intermetallic phase formation.
The effect of applied current intensity on the ignition time is shown in Table 1. At 100 A, no ignition was observed even after 600 s of current exposure. When current was increased from 200 to 300 A, the reaction ignition time was reduced from ~16.5 to ~5 s.
Figure 5 shows XRD patterns of the green compact and specimens exposed to 100, 200, and 300 A current intensity. No significant reaction took place at 100 A, even after 600 s of current exposure; hence, only aluminum and nickel peaks are present as was in the case of the green compact. The XRD patterns for specimens reacted under 200 and 300 A show no aluminum peaks, indicating the consumption of aluminum during the reaction. They also show the formation of intermetallic phases including Ni3Al, Al3Ni2, Al3Ni, and Ni5Al3. Moreover, residual nickel was also found in both cases.
Scanning electron microscopy (Fig. 6) in the backscattered mode of operation indeed shows a multiphase microstructure for specimens reacted under 200 and 300 A. The figure shows the microstructures under the tip location at the following regions: just beneath the initial aluminum-free nickel layer, mid thickness and close to the bottom of the reacted specimen for both 200 A (a, b, c) and 300 A (d, e, f). The figures show clear differences between specimens exposed to 200 and 300 A. Just under the initial aluminum-free nickel layer, for 300 A current exposure, the microstructure is more homogeneous and consolidated compared with the 200 A counterpart. This is believed to be a direct result of the higher currents/temperatures generated within this region under 300 A. This was also confirmed by investigating the initial aluminum-free Ni layer, where under 200 A close to the Ni/Al region, some minor aluminum was detected using EDXS (possibly through back infiltration of molten aluminum during the reaction from lower regions of the compact), but no tungsten was found. However, under 300 A current exposure, significant amounts of aluminum and tungsten were detected in the initial aluminum-free nickel layer due to the excessive diffusion of tungsten in nickel, indicating high temperature generation. Moreover, the tip had sunk into the specimen, giving rise to better consolidation within these regions. What is interesting is that at lower depths both specimen microstructures show inner (light) unreacted predominantly nickel regions; however, under 300 A larger such regions are observed, indicating a less complete reaction. This would at first appear contrary to expectations; however, if we consider the time the compact is allowed to heat prior to ignition, it would seem that under 200 A the compact has more time to pre-heat at larger distances away from the tip, prior to reaction ignition. Higher pre-heat temperatures of green compacts are typically known to improve homogeneity in SHS reactions due to an increase in the combustion temperature [9].
Under 300 A of current exposure, although an SHS reaction is expected to propagate after only ~5 s, it would propagate through a compact that has had less time for pre-heating, leading to a reduced combustion temperature and less complete reaction. Future study will be directed at implementing a setup that allows spatial and temporal temperature measurements to confirm these hypotheses. Interestingly, in Fig. 6a and f, it is clear that two district pore sizes are observed (large and small). The larger pores are surrounded by intermetallic phases which are brought upon by the reaction. The smaller pores exist within the predominantly nickel region, which indicate single-phase sintering of the elemental nickel powder. These types of pores are not clearly present in the microstructure within the mid-sections.
Figure 7 shows high magnification scanning electron micrographs taken from the mid thickness region of samples exposed to 200 and 300 A. These micrographs clearly show that the unreacted predominantly nickel regions are larger for the 300 A samples.
Figure 8 shows how the porosity changes across the thickness of the specimens for both 200 and 300 A samples. The results show that for both current exposures, the average residual porosity slightly increases away from the tip (although there is some error bar overlap). The figure also shows that under 300 A of current exposure, less porosity is generated than that under 200 A. The overall average porosity content at 200 and 300 A (an average of all spatial data points considered) are 23.2% ± 4.4 and 14.8% ± 5.8, respectively. Despite the lower overall pore content at 300 A, the average room temperature microhardness is slightly lower than that of the 200 A sample (143.2 Hv ± 20 and 152 Hv ± 33, respectively), although there is still some overlap when the error bars are accounted for. This is believed to be due to the presence of a larger amount of softer unreacted nickel in the microstructure of the 300 A reacted samples as compared to the 200 A reacted samples. In contrast, the average specimen microhardness for the 100 A current exposure was only 62 Hv ± 3, which is considerably lower than those of specimens reacted under 200 and 300 A. This is because under 100 A, no reaction had occurred and the microstructure contained basically unreacted mixtures of Ni and Al, as opposed to reactively formed intermetallic phases which possess higher hardnesses.
The results in this study show for the first time that it is possible to combustion synthesize Ni–Al powder mixture under local current activation using CATS. Future study will be directed at using moderate currents for longer times, investigation of the effect of environment (e.g., vacuum), green density, room temperature pressure transmitting medium, and multiple tips at pre-determined locations to promote a more uniform reaction “approaching” that of thermal explosion (volume combustion) reaction mode.
Conclusions
The following conclusions can be drawn from this study:
-
1.
R-CATS has been used for the first time to combustion synthesize Ni–Al macroscale intermetallics under an applied local current.
-
2.
Under the investigated processing conditions, an applied current intensity of 100 A did not trigger a combustion synthesis reaction even after 10 min of current exposure.
-
3.
Current intensities of 200 and 300 A resulted in a combustion synthesis reaction throughout the samples.
-
4.
Rapid formation of aluminum-rich Ni–Al intermetallic compounds under the 200 and 300 A imposed current intensity precedes and contributes to the ignition process.
-
5.
An increase in current intensity from 200 to 300 A results in a decrease in ignition time, microhardness, pore content, and microstructural homogeneity.
References
J. Gubicza, H.-Q. Bui, F. Fellah, N. Szasz, G. Dirras, Bulk ultrafine-grained nickel consolidated from nanopowder. Mater. Sci. Forum 589, 93 (2008)
U. Anselmi-Tamburini, S. Gennari, J.E. Garay, Z.A. Munir, Fundamental investigations on the spark plasma sintering/synthesis process II. Modeling of current and temperature distributions. Mater Sci Eng A A394(1–2), 139 (2005)
Cs. Balazsi, Z. Shen, Z. Konya, Zs. Kasztovszky, F. Weber, Z. Vertesy, L.P. Biro, I. Kiricsi, P. Aratoa, Processing of carbon nanotube reinforced silicon nitride composites by spark plasma sintering. Compos. Sci. Technol. 65(5), 727 (2005)
U. Anselmi-Tamburini, J.E. Garay, Z.A. Munir, Spark plasma sintering and characterization of bulk nanostructured fully-stabilized zirconia (FSZ): I. Densification studies. J. Mater. Sci. 19(11), 3255–3262 (2004)
Y.W. Gu, K.A. Khor, P. Cheang, Bone-like apatite layer formation on hydroxyapatite prepared by spark plasma sintering (SPS). Biomaterials 25(18), 4127 (2004)
S. Nemat-Nasser, Y. Su, W.-G. Guo, J. Isaacs, Experimental characterization and micromechanical modeling of super elastic response of a porous NiTi shape-memory alloy. J. Mech. Phys. Solids 53(10), 2320 (2005)
K. Morsi, K.S. Moon, S. Kassegne, R. Ugle, E. Villar, Novel current-activated tip-based sintering (CATS): localization of spark plasma sintering. Scr. Mater. 60, 745 (2009)
M. Patel, K.S. Moon, S.K. Kassegne, K. Morsi, Effects of current intensity and cumulative exposure time on the localized current-activated sintering of titanium nickelides. J. Mater. Sci. 46(20), 6690–6699 (2011)
K. Morsi, The diversity of combustion synthesis processing: a review. J. Mater. Sci. 47(1), 68–92 (2012)
A. Numula, K. Morsi, K.S. Moon, S.K. Kassegne, Exploratory investigations in reactive current activated tip-based sintering, in Proceedings of the 2011 TMS annual meeting, EPD congress, San Diego, CA, 2011, p. 819
J.E. Garay, U. Anselmi-Tamburini, Z.A. Munir, Enhanced growth of intermetallic phases in the Ni–Ti system by current effects. Acta Mater. 51(15), 4487 (2003)
Acknowledgments
The authors also wish to thank The National Science Foundation [CMMI division: Grant No. 0826532, Engineering Research Center: Grant No. 1028725, and major research instrumentation (MRI) Grant DBI-0959908] for their support. Thanks also to Dr. Steve Barlow, Mr. Greg Morris, and Mr. Mike Lester for assistance with scanning electron microscopy and general technical support, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Numula, A., Kassegne, S., Moon, K.S. et al. Reactive Current-Activated Tip-Based Sintering of Ni–Al Intermetallics. Metallogr. Microstruct. Anal. 2, 148–155 (2013). https://doi.org/10.1007/s13632-013-0071-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13632-013-0071-y