Abstract
Nosema apis and Nosema ceranae are gut parasites that infect western honey bees (Apis mellifera) worldwide. N. ceranae is an exotic infectious disease agent of A. mellifera, having been originally described in the Asian honey bee (Apis cerana), while N. apis is native to the western honey bee. To better understand the dynamics and epidemiology of the two pathogens, we examined the impact of European isolates of both Microsporidia on the longevity of European A. mellifera in a controlled laboratory experiment. N. ceranae caused slightly higher host mortality compared to N. apis, but differences in virulence were subtle and non-significant. Variation across published studies may reflect geographic differences in the coadaptation of hosts and parasites and seasonal differences in host susceptibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The western honey bee (Apis mellifera) is the most important commercially managed pollinator, with its services to agriculture estimated to exceed $200 billion per year (Klein et al. 2007; Gallai et al. 2009; Potts et al. 2010). Honey bee health is therefore a topic that has received considerable attention, especially given reports of dramatic and unexpected colony losses in the last decade (Carreck and Neumann 2010). Several factors have been suggested to account for the decrease in honey bee health status, especially the introduction and spread of pathogens due to beekeeping activities (Carreck and Neumann 2010; vanEngelsdorp and Meixner 2010; Williams et al. 2010; González-Varo et al. 2013; Vanbergen and the Insect Pollinators Initiative 2013). Of the wide range of pathogens infecting honey bees, several have been listed as high-risk stressors reducing individual host lifespan, with subsequent detrimental effects on the colony (reviewed by Genersch 2010; Evans and Schwarz 2011).
Many studies have focused on an exotic emerging pathogen, the Microsporidian Nosema ceranae (Higes et al. 2006; Chen et al. 2008; Fries 2010). First described in the Asian honey bee (Apis cerana, Fries et al. 1996), N. ceranae successfully infected the western honey bee, probably prior to 1997 (Paxton et al. 2007), and is nowadays globally distributed (Klee et al. 2007). In some regions, N. ceranae has been suggested to be replacing the native Nosema apis (Klee et al. 2007; Higes et al. 2013), the Microsporidian that was for years considered to be the only disease agent of nosemosis in western honey bees (Zander 1909; Bailey 1955). Our recent experimental data suggest that N. ceranae exhibits a strong priority effect and a competitive advantage over N. apis when infecting a worker bee before its congener, plausibly explaining its predominance (Natsopoulou et al. 2015).
Given the threat that Nosema spp. pose to honey bees, comparative studies investigating differences in host responses to both Microsporidia have been undertaken, often focusing on possible fitness costs to hosts, e.g. death as a measure of pathogen virulence of infection by N. ceranae versus N. apis. Both Microsporidia are obligate intracellular pathogens that infect the epithelial cells of the ventriculus of adult bees, resulting in gut tissue degeneration (Fries et al. 1996; Higes et al. 2007; Huang and Solter 2013). N. ceranae has been additionally associated with nutritional stress (Mayack and Naug 2009; Martín-Hernández et al. 2011) and immune suppression (Dussaubat et al. 2012; Aufauvre et al. 2014). Moreover, a direct link between N. ceranae infection and colony depopulation or even total loss has been shown in Spanish honey bee (Martín-Hernández et al. 2007; Higes et al. 2008, 2009, 2010; Botías et al. 2013). However, in other regions of the world, colony loss due to N. ceranae infection has not been reported (Cox-Foster et al. 2007; vanEngelsdorp et al. 2009; Gisder et al. 2010); the impact of N. ceranae may indeed vary across countries and climatic conditions.
At the level of the individual honey bee, studies are contradictory concerning the virulence of N. ceranae over N. apis. Paxton et al. (2007), Martín-Hernández et al. (2011) and Williams et al. (2014) found that N. ceranae induced higher mortality than N. apis, but this was not confirmed by Forsgren and Fries (2010), Huang et al. (2015) or Milbrath et al. (2015), who found the two Microsporidia species to induce similar mortality. The experiments by Forsgren and Fries (2010) and Milbrath et al. (2015) were not designed focusing on host mortality, and authors did not comment in detail on the lack of differences in host mortality induced by the two Microsporidia. Huang et al. (2015), on the other hand, explicitly tested for differences in virulence; they (Huang et al. 2015) hypothesised that adaptation of A. mellifera to N. ceranae may have already occurred in North America (but not in Europe), which could explain why North American honey bees exhibit similar mortality to N. apis and N. ceranae, in contrast to Europe, where N. ceranae seems to be more virulent than N. apis (Paxton et al. 2007; Martín-Hernández et al. 2011).
Because of these contradictory results and the still open question of the possibly greater virulence of the exotic N. ceranae over the native N. apis, we test the hypothesis of Huang et al. (2015) that N. ceranae is more virulent than N. apis for honey bees in Europe. Studies of comparative virulence may also help to explain why N. ceranae has become dominant over N. apis in warmer regions of the world, and if the variation in virulence reported in the literature is a reflection of differences in geographic coadaptation of hosts and Microsporidia. Specifically, we performed mortality bioassays using honey bee workers originating from Germany in which bees were experimentally infected with European isolates of either N. apis or N. ceranae, and host fitness (parasite virulence) in terms of host longevity was monitored in carefully controlled cage experiments.
2 Material and methods
2.1 Host and pathogen preparation
Three queen-right, unrelated colonies of A. mellifera (typical local Halle (Germany) beekeeper colonies of the subspecies A. mellifera carnica) were used as the source of worker bees. Bees that newly emerged in the laboratory were placed in metal cages, with bees from source colonies equally mixed across cages (18 bees per cage) so as to eliminate any colony genotypic effect, and then kept for 3 days in an incubator at +30 °C with ad libitum 50 % (w/v) sucrose solution following standard procedures (Williams et al. 2013) until inoculation.
Spores of both Nosema species were obtained from artificial propagations kept in the lab through mass feeding of caged adult honey bees. N. ceranae spores originated from Germany, while N. apis spores originated from Sweden. On the day of infection, several (>15) host bees per propagation were checked under the microscope in order to verify inoculation success; each bee contained millions of spores. Freshly prepared inocula containing spores of either N. ceranae or N. apis were obtained by crushing the ventriculus of honey bees from the propagations in distilled water and purified by triangulation (Fries et al. 2013). Nosema spores were counted using a Fuchs-Rosenthal haemocytometer. Spore solutions were kept at room temperature throughout the whole procedure until inoculation of all experimental bees on the same day (maximum 8 h between extractions from propagation bees to feeding to experimental bees). The same procedure was followed using uninfected caged bees that served as control inoculum.
2.2 Inoculation
Four-day-old caged workers were individually fed with 10 μl of 50 % w/v sucrose solution containing a control solution, or 105 spores of either N. ceranae or N. apis: this dosage has been repeatedly shown to result in 100 % Nosema infection in honey bees (Forsgren and Fries 2010; Huang et al. 2015). For inoculations, bees were immobilised by hand following the recommendations of Human et al. (2013) without prior anaesthesia; attention was given to avoid any physical harm to hosts. After feeding, each individual was kept isolated in a 1.5-mL Eppendorf tube for ca. 30 min post infection to avoid any pathogen exchange through trophallaxis (Fries et al. 2013). Bees that did not consume the entire volume of inoculum during feeding or were observed to have discharged the inoculum during the first 30 min were discarded. Each treatment was replicated five times (five independent replicate cages) with 18 bees per cage. All replicates and all treatments were set up within the same day. Cages were placed back in the incubator and checked every 24 h starting the day after inoculation. Dead bees were counted and removed from each cage. Observations continued until all bees in all replicates were dead.
Absence of cross-contamination in the inocula used for the infections was confirmed with a multiplex PCR as described in Fries et al. (2013). To verify if the infection had been successful, bees that died at 21 days post infection (p.i.) were individually checked under the microscope for the presence of Nosema spp. At that age, both species have already asymptoted in number in the ventriculus (Forsgren and Fries 2010; Huang and Solter 2013). DNA was also extracted from these bees (pooled per treatment) and, using the same multiplex PCR described above, they were checked for cross-contamination.
2.3 Survival analysis
Survival analysis was performed in R v 3.1.0 using a mixed effects Cox proportional hazard model (R package ‘survival’ (Therneau and Grambsch 2000), and the R package ‘coxme’ (Therneau et al. 2003)), with treatment as a fixed factor and cage (replicate) as a random effect. Including cage as a random effect did not improve the model (P > 0.05), indicating that there was no significant variation between replicates. It was nevertheless included in the model as an integral component of the experimental design. Post hoc analysis to test significant differences between treatment means was performed using the R package ‘multcomp’ (Hothorn et al. 2007), applying a Bonferroni correction for multiple testing.
We used two approaches to evaluate the statistical power with which differences in host mortality between Microsporidia species could be detected. Firstly, given our sample sizes and the variation we observed in our dataset, we increased the difference in mean survival between the two treatments by reducing the survival of the N. ceranae treatment group while keeping that of the N. apis treatment group constant, then tested for significance of differences in survival. Secondly, we estimated the power of our current analysis (ß) and the sample size of host individuals necessary to increase statistical power so as to be able to reject the null hypothesis of no difference between Nosema spp. treatments using the R package ‘powerSurvEpi’ (Qiu et al. 2012).
3 Results
PCR demonstrated that inocula fed to bees contained only N. ceranae or N. apis, while control solutions were devoid of Microsporidia DNA and, by visual inspection, they were devoid of spores, too. Microscopic analysis of individual bees that died 21 days p.i. showed that infections were successful as Nosema spores were observed across all replicate cages in both Microsporidia treatments (n = 8 individual bees per treatment). Spores were not observed in control bees (n = 5) and PCRs on the same bees (pooled per treatment) verified the absence of cross-contamination.
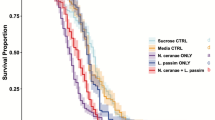
Nosema treatments resulted in slightly but significantly faster mortality of caged honey bees (Figure 1). During the first 10 days p.i., mortality was slight and below 10 % across all treatments (Figure 1). The median survival time (LT50) was 23 days p.i. (95 % CI, 22–24) for the control, 20 days p.i. (19–22) for N. ceranae and 21 days p.i. (20–22) for N. apis treatments. Treatment was a significant predictor of survival; when it was included as an explanatory variable, the statistical model was significantly better compared to the null model without treatment (likelihood ratio χ 2 = 16.8, df = 2, P < 0.05). Though infection with N. ceranae or N. apis significantly increased mortality compared to the controls (hazard ratio:control vs. N. ceranae:z = 3.44, P < 0.05, control vs. N. apis:z = 3.51, P < 0.05), survival did not differ significantly between Microsporidian treatments N. apis and N. ceranae (P > 0.05).
Our power analysis showed that, given our samples size and the variance detected in our dataset, if differences in mean survival of the two Microsporidia treatments had been >3 days, the difference would have been significant. Differences in virulence that we observed were clearly less than 3 days. Indeed, the statistical power (ß) of our survival analysis given the observed differences in hazard ratio was found to be very low (0.03), indicating that small differences in hazard ratio, as we found, need a great number of replicates in order to detect a difference as statistically significant (estimated sample size, >15.000 individuals per treatment). Overall, our power analyses suggest that the observed differences in virulence between Microsporidia species were minimal in our experimental paradigm.
4 Discussion/conclusion
Our experiment confirms that Nosema spp. are virulent for their host A. mellifera; infection significantly decreased longevity of honey bee workers, albeit only by 2–3 days in our laboratory set-up. Yet, we found only slight and statistically non-significant differences in virulence (mortality) between N. ceranae and N. apis. We can thus reject the hypothesis of Huang et al. (2015) that honey bees in Europe respond different to N. ceranae infection versus N. apis infection; we find that honey bees in Europe respond similarly to N. ceranae infection and N. apis infection, as they do in USA (Huang et al. 2015).
These results contrast with others from Europe (Paxton et al. 2007, a coauthor of the current study; Martín-Hernández et al. 2011) and Canada (Williams et al. 2014), in which N. ceranae was found to be much more virulent than N. apis for caged honey bees. Rather, our results correspond more closely with the recently reported results from the USA showing that N. ceranae is not more virulent that N. apis in terms of inducing host mortality (Huang et al. 2015; Milbrath et al. 2015). Huang et al. (2015) argue that differences between their results and those reported from Europe may reflect an adaption of USA bees to N. ceranae through long-term exposure and thus reduced susceptibility, or lower virulence of USA isolates of N. ceranae. Our data show that the same host responses can be observed in European bees and thus the similarity in virulence of N. apis and N. ceranae found by Huang et al. (2015) does not represent a regional effect found only in North American honey bees and North American isolates of Microsporidia.
One explanation for the variability across published studies is that differences in virulence between the two Microsporidian species are sensitive to experimental conditions. We consider this unlikely because most reported studies employ standard experimental approaches in which honey bee workers are individually fed freshly harvested spores of Nosema (Fries et al. 2013) and are then caged en masse (Williams et al. 2013).
Variation exists across published studies in the age at which bees are inoculated, variation that could potentially explain differences in results across studies. Differences in susceptibility among bees infected at different ages has been demonstrated by Huang et al. (2015), who found newly emerged bees (<24 h after eclosion) to be less susceptible to both Nosema spp., particularly to N. ceranae. Yet, studies using same-age bees have generated inconsistent results: Williams et al. (2014) and Milbrath et al. (2015) each inoculated bees a few hours after eclosion (<24 h) and reported higher virulence of N. ceranae or no difference between the two species, respectively. Martín-Hernández et al. (2011) and Huang et al. (2015) both infected 5-day-old bees in their experiments but also found contradictory results, with N. ceranae more virulent or no different in virulence, respectively. Differences in study protocols and age of bees at infection therefore seem unlikely to explain differences in study results. However, extensive studies on the interaction between age-dependent susceptibility to infection and host genotype are lacking. In our study, we followed procedures to minimise handling effects on bees and ensure high spore viability in both inocula by employing standard experimental approaches (Fries et al. 2013; Human et al. 2013; Williams et al. 2013); we acknowledge, though, that differences in spore viability coupled to length of host exposure might also explain our results.
The discrepancy between our results and those of previous studies in Europe may alternatively reflect different responses of the host due to genetic differences of either the host or the pathogen. As host genotype is considered a key factor determining infection outcome (Minchella 1985; de Roode et al. 2004), different host subspecies may vary in their ability to counter infection. At least 26 subspecies of A. mellifera have been described (Ruttner et al. 1978), and variation in response to Nosema spp. may lie in inherent host subspecific differences. For example, experiments in Spain are conducted using A. mellifera iberiensis (Higes et al. 2007) while A. mellifera mellifera, A. mellifera carnica or Buckfast (a cross of many subspecies) are usually tested in central and northern Europe (Mulholland et al. 2012; Fontbonne et al. 2013; Francis et al. 2014). However, recent studies suggest that genetic variation among individual bees or colonies within a subspecies—and not between subspecies—is a better predictor of host response to N. ceranae (Fontbonne et al. 2013; Francis et al. 2014; Meixner et al. 2014).
Genetic differences among pathogen isolates from different geographic locations may also lead to variation in response of the host. N. ceranae in Spain has been associated with colony depopulation and colony losses and a higher prevalence than N. apis (Higes et al. 2008; Fernández et al. 2012). In Germany, both Nosema species co-exist in populations at a similar prevalence (Gisder et al. 2010), with no impact on colony losses (Genersch 2010; Gisder et al. 2010). This suggests the presence in Europe of N. ceranae strains that differ in virulence. However, a study comparing N. ceranae strains from France and Spain failed to show any difference, either genetically in the parasite or in terms of virulence, measured as the mortality it induced in host worker honey bees (Dussaubat et al. 2013). A recent genomic study also supports the idea that N. ceranae does not differ across the range of A. mellifera and exists as a single clonal variant (Pelin et al. 2015).
Though we have argued that genetic differences among host subspecies or pathogen isolates are unlikely to account for differences observed in studies of the virulence of N. apis versus N. ceranae, specific combinations of host-pathogen genotypes might nevertheless lead to deferent infection outcome. Interaction effects between host and parasite genotypes (G × G) have been found to play a key role determining infection traits such as virulence in several host-parasite systems (Lambrechts et al. 2006). Moreover, genetic differences may lead to differences in virulence if they are dependent on environment (e.g. climatic conditions), known as a genotype × environment interaction (Thomas and Blanford 2003; Wolinska and King 2009). This notion may explain why N. ceranae in Spain induced 100 % host mortality at 8 days post infection (Higes et al. 2007; Martín-Hernández et al. 2011), whereas in Sweden and Canada, it induced <25 % host mortality in the same time frame (Forsgren and Fries 2010; Williams et al. 2014). Whether the response of hosts to N. apis varies similarly is not known.
Worker honey bee physiology varies profoundly across the year in temperate regions of the world (Winston 1987), and this variation may potentially be of greater importance than genotype × environment interactions in explaining differences among published results in virulence of pathogens. For example, Doublet et al. (2015) found mortality induced by N. ceranae to exhibit a strong seasonal effect. If the susceptibility of honey bees to N. apis and N. ceranae varies differentially across the year, this might also explain differences observed between studies in the relative virulence of N. apis and N. ceranae.
That we did not find a significant difference between the two pathogens in inducing mortality in our experiment may not be surprising as the size of the effect was small; the median survival time of bees infected with N. ceranae was only 1 day less than that of N. apis (20 vs. 21 days for N. ceranae and N. apis respectively). As we have shown, small effect sizes require greater experimental replication to detect differences. We note that another recent study with European honey bees also found N. ceranae to induce a slightly higher, albeit non-significant, mortality than N. apis (Gajda et al. 2014).
Of course, subtle differences in the response of caged honey bees in a benign environment may translate into more profound differences in behaviour or longevity in a harsh field environment (e.g. homing ability, Wolf et al. 2014). We cannot therefore predict what the impact of the small and insignificant difference we observed in our cage experiment might be under natural conditions, where worker honey bees face more stressful conditions. However, the successful spread of N. ceranae and its dominance over N. apis in some locations is probably more related to the competitive advantage of N. ceranae over N. apis in warmer regions of the world (Natsopoulou et al. 2015) rather than to its higher virulence in terms of inducing host mortality. That our results concur with those from the USA (Huang et al. 2015; Milbrath et al. 2015) in showing that N. ceranae differs little in virulence from N. apis highlights the necessity for future research to focus on exploring other mechanisms responsible for shaping the current patterns of distribution and prevalence of N. ceranae and N. apis, such as comparative transmission dynamics, parasite inter-species competition and host genotype × parasite genotype effects.
References
Aufauvre, J., Misme-Aucouturier, B., Viguès, B., Texier, C., Delbac, F., Blot, N. (2014) Transcriptome analyses of the honeybee response to Nosema ceranae and insecticides. PLoS One. 9 (3), e91686
Bailey, L. (1955) The epidemiology and control of Nosema disease of the honey-bee. Ann. Appl. Biol. 43(3), 379–389
Botías, C., Martín-Hernández, R., Barrios, L., Meana, A., Higes, M. (2013) Nosema spp. infection and its negative effects on honey bees (Apis mellifera iberiensis) at the colony level. Vet Res 44(25), 1–14
Carreck, N., Neumann, P. (2010) Honey bee colony losses. J. Apic. Res. 49(1), 1–6
Chen, Y., Evans, J.D., Smith, I.B., Pettis, J.S. (2008) Nosema ceranae is a long-present and wide-spread microsporidian infection of the European honey bee (Apis mellifera) in the United States. J. Invertebr. Pathol. 97(2), 186–188
Cox-Foster, D.L., Conlan, S., Holmes, E.C., Palacios, G., Evans, J.D., et al. (2007) A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318(5848), 283–287
de Roode, J.C., Culleton, R., Cheesman, S.J., Carter, R., Read, A.F. (2004) Host heterogeneity is a determinant of competitive exclusion or coexistence in genetically diverse malaria infections. Proc. Biol. Sci. 271(1543), 1073–1080
Doublet, V., Labarussias, M., de Miranda, J.R., Moritz, R.F.A., Paxton, R.J. (2015) Bees under stress: sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ. Microbiol. 17(4), 969–983
Dussaubat, C., Brunet, J.L., Higes, M., Colbourne, J.K., Lopez, J., et al. (2012) Gut pathology and responses to the microsporidium Nosema ceranae in the honey bee Apis mellifera. PLoS One. 7 (5), e37017
Dussaubat, C., Sagastume, S., Gómez-Moracho, T., Botías, C., García-Palencia, P., Martín-Hernández, R., Le Conte, Y., Higes, M. (2013) Comparative study of Nosema ceranae (Microsporidia) isolates from two different geographic origins. Vet. Microbiol. 162(2–4), 670–678
Evans, J.D., Schwarz, R.S. (2011) Bees brought to their knees: microbes affecting honey bee health. Trends Microbiol. 19(12), 614–620
Fernández, J.M., Puerta, F., Cousinou, M., Dios-Palomares, R., Campano, F., Redondo, L. (2012) Asymptomatic presence of Nosema spp. in Spanish commercial apiaries. J. Invertebr Pathol 111(2), 106–110
Fontbonne, R., Garnery, L., Vidau, C., Aufauvre, J., Texier, C., Tchamitchian, S., El Alaoui, H., Brunet, J.L., Delbac, F., Biron, D.G. (2013) Comparative susceptibility of three Western honeybee taxa to the microsporidian parasite Nosema ceranae. Infect. Genet. Evol. 17, 188–194
Forsgren, E., Fries, I. (2010) Comparative virulence of Nosema ceranae and Nosema apis in individual European honey bees. Vet. Parasitol. 170(3–4), 212–217
Francis, R.M., Amiri, E., Meixner, M.D., Kryger, P., Gajda, A., et al. (2014) Effect of genotype and environment on parasite and pathogen levels in one apiary - a case study. J. Apic. Res. 53(2), 230–232
Fries, I. (2010) Nosema ceranae in European honey bees (Apis mellifera). J. Invertebr. Pathol. 103(Suppl), S73–S79
Fries, I., Feng, F., da Silva, A., Slemenda, S.B., Pieniazek, N.J. (1996) Nosema ceranae n. sp. (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). Eur. J Protistol 32(3), 356–365
Fries, I., Chauzat, M.P., Chen, Y.P.P., Doublet, V., Genersch, E., et al. (2013) Standard methods for Nosema research. J. Apic. Res. 52(1), 1–28
Gajda, A.M., Topolska, G., Grzęda, U., Czopowicz, M. (2014) Nosema ceranae in interaction with Nosema apis and Black Queen Cell Virus, in: European Conference of Apidologie (EurBee). Murcia, Spain, p. 33
Gallai, N., Salles, J.M., Settele, J., Vaissière, B.E. (2009) Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 68(3), 810–821
Genersch, E. (2010) Honey bee pathology: current threats to honey bees and beekeeping. Appl. Microbiol. Biotechnol. 87(1), 87–97
Gisder, S., Hedtke, K., Möckel, N., Frielitz, M.C., Linde, A., Genersch, E. (2010) Five-year cohort study of Nosema spp. in Germany: does climate shape virulence and assertiveness of Nosema ceranae? Appl. Environ Microbiol 76(9), 3032–3038
González-Varo, J.P., Biesmeijer, J.C., Bommarco, R., Potts, S.G., Schweiger, O., Smith, H.G., Steffan-Dewenter, I., Szentgyörgyi, H., Woyciechowski, M., Vilà, M. (2013) Combined effects of global change pressures on animal-mediated pollination. Trends Ecol. Evol. 28(9), 524–530
Higes, M., Martín, R., Meana, A. (2006) Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J. Invertebr. Pathol. 92(2), 93–95
Higes, M., García-Palencia, P., Martín-Hernández, R., Meana, A. (2007) Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 94(3), 211–217
Higes, M., Martín-Hernández, R., Botías, C., Bailón, E., González-Porto, A., et al. (2008) How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 10(10), 2659–2669
Higes, M., Martín-Hernández, R., Garrido-Bailón, E., González-Porto, A.V., García-Palencia, P., Meana, A., Del Nozal, M.J., Mayo, R., Bernal, J.L. (2009) Honeybee colony collapse due to Nosema ceranae in professional apiaries. Environ. Microbiol. Rep. 1(2), 110–113
Higes, M., Martín-Hernández, R., Martínez-Salvador, A., Garrido-Bailón, E., González-Porto, A.V., Meana, A., Bernal, J.L., Del Nozal, M.J., Bernal, J. (2010) A preliminary study of the epidemiological factors related to honey bee colony loss in Spain. Environ. Microbiol. Rep. 2(2), 243–250
Higes, M., Meana, A., Bartolomé, C., Botías, C., Martín-Hernández, R. (2013) Nosema ceranae (Microsporidia), a controversial 21st century honey bee pathogen. Environ. Microbiol. Rep. 5(1), 17–29
Hothorn, T., Bretz, F., Westfall, P., Heiberger, R.M. (2007) Multcomp: Simultaneous Inference for General Linear Hypotheses, URL http://www.R-project.org/
Huang, W.-F., Solter, L.F. (2013) Comparative development and tissue tropism of Nosema apis and Nosema ceranae. J. Invertebr. Pathol. 113(1), 35–41
Huang, W.-F., Solter, L., Aronstein, K., Huang, Z. (2015) Infectivity and virulence of Nosema ceranae and Nosema apis in commercially available North American honey bees. J. Invertebr. Pathol. 124, 107–113
Human, H., Brodschneider, R., Dietemann, V., Dively, G., Ellis, J.D., et al. (2013) Miscellaneous standard methods for Apis mellifera research. J. Apic. Res. 52(4), 1–56
Klee, J., Besana, A.M., Genersch, E., Gisder, S., Nanetti, A., et al. (2007) Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invertebr. Pathol. 96(1), 1–10
Klein, A.M., Vaissière, B.E., Cane, J.H., Steffan-Dewenter, I., Cunningham, S.A., Kremen, C., Tscharntke, T. (2007) Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 274(1608), 303–313
Lambrechts, L., Fellous, S., Koella, J.C. (2006) Coevolutionary interactions between host and parasite genotypes. Trends Parasitol. 22(1), 12–16
Martín-Hernández, R., Meana, A., Prieto, L., Salvador, A.M., Garrido-Bailón, E., Higes, M. (2007) Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 73(20), 6331–6338
Martín-Hernández, R., Botías, C., Barrios, L., Martínez-Salvador, A., Meana, A., Mayack, C., Higes, M. (2011) Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol. Res. 109(3), 605–612
Mayack, C., Naug, D. (2009) Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 100(3), 185–188
Meixner, M.D., Francis, R.M., Gajda, A., Kryger, P., Andonov, S., et al. (2014) Occurrence of parasites and pathogens in honey bee colonies used in a European genotype-environment interactions experiment. J. Apic. Res. 53(2), 215–219
Milbrath, M., van Tran, T., Huang, W.-F., Solter, L.F., Tarpy, D.R., Lawrence, F., Huang, Z.Y. (2015) Comparative virulence and competition between Nosema apis and Nosema ceranae in honey bees (Apis mellifera). J. Invertebr. Pathol. 125, 9–15
Minchella, D.J. (1985) Host life-history variation in response to parasitism. Parasitology 90(1), 205–216
Mulholland, G., Traver, B., Johnson, N., Fell, R. (2012) Individual variability of Nosema ceranae infections in Apis mellifera colonies. Insects 3(4), 1143–1155
Natsopoulou, M.E., McMahon, D.P., Doublet, V., Bryden, J., Paxton, R.J. (2015) Interspecific competition in honeybee intracellular gut parasites is asymmetric and favours the spread of an emerging infectious disease. Proc. Biol. Sci. 282, doi:10.1098/rspb.2014.1896
Paxton, R., Klee, J., Korpela, S., Fries, I. (2007) Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 38, 558–565
Pelin, A., Selman, M., Aris-Brosou, S., Farinelli, L., Corradi, N. (2015) Genome analyses suggests the presence of polyploidy and recent human-driven expansions in eight global populations of the honeybee pathogen Nosema ceranae. Environ. Microbiol. . doi:10.1111/1462-2920.12883
Potts, S.G., Biesmeijer, J.C., Kremen, C., Neumann, P., Schweiger, O., Kunin, W.E. (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25(6), 345–353
Qiu, W., Chavarro, J., Lazarus, R., Rosner, B., Ma, J. (2012) powerSurvEpi: Power and sample size calculation for survival analysis of epidemiological studies, URL http://www.R-project.org/
Ruttner, F., Tassencourt, L., Louveaux, J. (1978) Biometrical-statistical analysis of the geographic variability of Apis mellifera L. Apidologie 9(4), 363–381
Therneau, T.M., Grambsch, P.M. (2000) Modeling Survival Data: Extending the Cox Model. Springer, New York
Therneau, T.M., Grambsch, P.M., Pankratz, V. (2003) Penalized survival models and frailty. J. Comput. Graph. Stat. 12(1), 156–175
Thomas, M.B., Blanford, S. (2003) Genetic variation in a host-parasite association: potential for coevolution and frequency-dependent selection. Trends Ecol. Evol. 18(7), 344–350
Vanbergen, A.J., the I.P. Initiative (2013) Threats to an ecosystem service: pressures on pollinators. Front. Ecol. Environ. 11(5), 251–259
vanEngelsdorp, D., Evans, J.D., Saegerman, C., Mullin, C., Haubruge, E., et al. (2009) Colony collapse disorder: a descriptive study. PLoS One. 4 (8), e6481
vanEngelsdorp, D., Meixner, M.D. (2010) A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 103(Suppl), S80–S95
Williams, G.R., Tarpy, D.R., vanEngelsdorp, D., Chauzat, M.P., Cox-Foster, D.L., Delaplane, K.S., Neumann, P., Pettis, J.S., Rogers, R.E.L., Shutler, D. (2010) Colony collapse disorder in context. Bioessays 32(10), 845–846
Williams, G.R., Alaux, C., Costa, C., Csáki, T., Doublet, V., et al. (2013) Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J. Apic. Res. 52(1), 1–36
Williams, G.R., Shutler, D., Burgher-MacLellan, K.L., Rogers, R.E.L. (2014) Infra-population and -community dynamics of the parasites Nosema apis and Nosema ceranae, and consequences for honey bee (Apis mellifera) hosts. PLoS One. 9 (7), e99465
Winston, M. (1987) The Biology of the Honey Bee. Harvard University Press, Massachusetts and London
Wolf, S., McMahon, D.P., Lim, K.S., Pull, C.D., Clark, S.J., Paxton, R.J., Osborne, J.L. (2014) So near and yet so far: harmonic radar reveals reduced homing ability of Nosema infected honeybees. PLoS One. 9 (8), e103989
Wolinska, J., King, K.C. (2009) Environment can alter selection in host-parasite interactions. Trends Parasitol. 25(5), 236–244
Zander, E. (1909) Tierische Parasiten als Krankenheitserreger bei der Biene. Münchener Bienenzeitung 31, 196–204
Acknowledgments
We thank Christopher Mayack for assistance in the laboratory, Ingemar Fries for supplying N. apis spores and the anonymous referees for very useful comments which helped improve the manuscript. This work was supported by the Federal Ministry of Food, Agriculture and Consumer Protection (Germany): Fit Bee project (grant 511-06.01-28-1-71.007-10).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript editor: Peter Rosenkranz
Des isolats européens des microsporidies Nosema apis et Nosema ceranae présentent la même virulence vis-à-vis des ouvrières de l’abeille européenne dans des essais en laboratoire
Apis mellifera / relation hôte parasite / survie / Microsporidia
Europäische Isolate der Mikrosporidienarten Nosema apis und Nosema ceranae zeigen in Labortests ähnliche Virulenz gegenüber Arbeiterinnen der europäischen Honigbiene
Apis mellifera / Microsporidien / Wirt / Parasit / Überlebenszeit
Rights and permissions
About this article
Cite this article
Natsopoulou, M.E., Doublet, V. & Paxton, R.J. European isolates of the Microsporidia Nosema apis and Nosema ceranae have similar virulence in laboratory tests on European worker honey bees. Apidologie 47, 57–65 (2016). https://doi.org/10.1007/s13592-015-0375-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-015-0375-9