Abstract
Introduction
A growing number of biologics have recently been approved in China for psoriasis treatment, and some of these are eligible for Chinese medical insurance, resulting in a significant increase in the number of patients receiving these biologics. Nevertheless, real-world data on the efficacy and safety of biologics for treating moderate-to-severe plaque psoriasis in Chinese patients are limited, and relevant pharmacoeconomic studies are lacking. Therefore, we performed a prospective, single-center study to evaluate the efficacy and safety of adalimumab (ADA) and secukinumab (SEC) in real-world practice. A cost-effectiveness analysis (CEA) was also conducted.
Methods
Participants were enrolled between January 2019 and December 2020 at the West China Hospital, Sichuan University. Baseline and follow-up assessments were conducted, and an appropriate statistical analysis was performed.
Results
A total of 183 patients were included. At week 12, the number of patients achieving a psoriasis area and severity index reduction of 75% (PASI 75) with SEC treatment was higher than that with ADA and methotrexate (MTX) (SEC versus ADA versus MTX, 90.59% versus 58.70% versus 17.14%, respectively). Adverse events (AEs) were reported in 44.83% and 56.36% of patients in the SEC and ADA groups, respectively. The cost-effectiveness ratio in the SEC group was 46,311.83 Chinese yuan(CNY), compared with 17,580.92 CNY in the ADA group.
Conclusion
In real-world practice, SEC and ADA are effective and safe for moderate-to-severe plaque psoriasis treatment in Chinese patients. On the basis of drug prices during our study period without considering access to health insurance, ADA was more cost-effective in real-world practice.
Plain Language Summary
Adalimumab and secukinumab are two monoclonal antibodies used for the treatment of psoriasis, which target different cytokines in the pathogenesis. A growing number of biologics have recently been approved in China for psoriasis treatment including adalimumab and secukinumab, which are eligible for Chinese medical insurance, resulting in a significant increase in the number of patients receiving these biologics. With the purpose of evaluating its efficacy and safety in the real world, we registered the data of eligible patients in West China Hospital, Sichuan University over the past two years and conducted statistical analysis. In order to provide different therapeutic strategies for patients based on case-specific needs and access to financial resources, we performed pharmacoeconomic analyses to evaluate the cost-effectiveness of the two drugs. Our study demonstrated that adalimumab and secukinumab were effective and safe for moderate-to-severe plaque psoriasis in Chinese patients in the real-world practice. Based on drug prices during our study period and without taking into consideration access to health insurance, ADA was more cost-effective in real-world practice.
Similar content being viewed by others
Why carry out this study? |
A growing number of biologics have been approved for psoriatic treatment, and some of these are covered by the Chinese medical insurance. Nevertheless, real-world data on the efficacy and safety of biologics and relevant research in pharmacoeconomics are limited in China. |
We aimed to contribute real-world evidence of the efficacy and safety of adalimumab (ADA) and secukinumab (SEC) and recommended different therapeutic strategies for patients with different requirements. |
What was learned from the study? |
In real-world practice, ADA and SEC are effective and safe for treating moderate-to-severe plaque psoriasis in Chinese patients. On the basis of the drug prices during our study period without considering access to health insurance, ADA was more cost-effective than SEC. Among patients with different needs, SEC might be a better option for those who can afford its higher cost and desire better skin clearance and higher quality of life. |
We recommend different therapeutic strategies for patients based on case-specific needs and access to financial resources. |
Introduction
Psoriasis is a chronic immune-mediated inflammatory disease (IMID). According to the World Psoriasis Day consortium, there are approximately 125 million patients with psoriasis worldwide [1], with a prevalence of psoriasis in adults between 0.51% and 11.43% [2]. In 2012, an epidemiological investigation among six provinces in China suggested that the prevalence of psoriasis in China was 0.47%, with no difference in gender [3]. As a common and systemic disease, psoriasis can be accompanied by various comorbidities, including psoriatic arthritis (PsA), cardiovascular diseases, metabolic syndrome, inflammatory bowel disease, and psychiatric disorders [4]. For most patients, psoriasis causes distinct impairment to their visual appearance often resulting in social discrimination, thereby negatively impacting their life quality.
Fortunately, over the past two decades, significant breakthroughs have been made in treating psoriasis using biologics. For instance, a clinical trial showed that 57.9% of psoriasis patients treated with adalimumab (ADA) achieved a psoriasis area and severity index reduction of 75% (PASI 75) at week 16, while less than 40% did so in the methotrexate (MTX) group [5]. Moreover, a network meta-analysis demonstrated that more than 80% of patients using infliximab, secukinumab (SEC), and ixekizumab were found to have reached PASI 75 at their 10–16 weeks follow-up assessment [6]. On the basis of existing evidence, biologics for treating moderate-to-severe psoriasis have been codified in multiple national guidelines [7, 8]. Recently in China, many biological agents have been approved—including tumor necrosis factor alpha (anti-TNF-α) and interleukin 17 inhibitor (anti-IL-17)—of which ADA and SEC were the earliest and most widely used. However, studies on the efficacy and safety of ADA and SEC in clinical use are limited in the Chinese psoriasis population.
Despite the remarkable efficacy of biologics, their high cost affects decision-making regarding the economic value of different treatment options. Thus, we compared the efficacy and safety, and pharmacoeconomic status, of ADA and SEC for the treatment of moderate-to-severe plaque psoriasis in the Chinese population and provided relevant data for their use in clinical practice.
Methods
Study Design
A single-center, prospective, observational cohort study was conducted at the Department of Dermatology and Venereology, West China Hospital, Sichuan University from 1 January 2019 to 31 December 2020.
Ethics
This study was approved by the ethics committee of West China Hospital of Sichuan University (approval number 2020-234) and conducted in accordance with the Declaration of Helsinki (2000 EDITION) and/or all applicable federal and local regulations. Prior to enrollment, each patient signed a written informed consent form.
Study Population
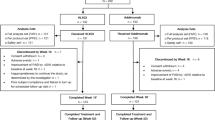
Eligible patients were 18 years of age or older diagnosed with moderate-to-severe plaque psoriasis with or without PsA at baseline who were prescribed ADA, SEC, or MTX with informed consent. The following criteria defined moderate-to-severe plaque psoriasis: (1) a psoriasis area and severity index (PASI) score of 3 or higher in a range from 0 to 72, with higher scores indicating more severe symptoms; (2) at least 3% of body surface area (BSA) affected by psoriasis or a dermatology life quality index (DLQI) score of no less than 6 [9]. Patients allergic to biologics or MTX, or with active tuberculosis or other severe infectious diseases, severe heart, lung, liver, kidney, or other vital organ system diseases, as well as neurological or mental disorders, were excluded. Pregnant or lactating women and women of childbearing age with a pregnancy plan were also excluded from the study. Patients without adequate data at baseline and at weeks 4 and 12 were also omitted from the final analyses.
Treatment
Patients were assigned nonrandomly to the ADA (n = 55), SEC (n = 87), or MTX (n = 41) groups according to their disease severity, screening results of biologics, and shared discussions between doctors and patients. ADA was administered as two injections of 40 mg (80 mg total) at week 0 and one injection of 40 mg at week 1, with subsequent injections every 2 weeks. SEC was administered as two injections of 150 mg (300 mg total) at weeks 0, 1, 2, 3, and 4, and every 4 weeks thereafter. MTX was administered as oral tablets 7.5–15 mg per week.
Assessments and Outcomes
Patient characteristics, PASI score, BSA, Physician Global Assessment (PGA) and DLQI scores, laboratory tests, and drug usage were collected during the study period. Disease severity assessment and safety follow-up were conducted at weeks 4 and 12 after treatment was initiated. The primary endpoints were the rate of PASI 75, PGA score of 0 or 1, and DLQI score of 0 or 1 (range of 0–30, with higher scores indicating a more significant effect of psoriasis on daily life) at week 12. Safety assessments include all adverse events (AEs), serious adverse events (SAEs), laboratory values such as routine blood test, aminopherase, serum creatinine, and QuantiFERON-TB Gold, et al, at weeks 4 and 12, and routine evaluation of vital signs.
Pharmacoeconomic Analyses
The cost-effectiveness analysis (CEA) method shown in Eq. (1)—where C is the cost of biologics per person in the first 3 months, and E is the percentage of PASI 75 responsiveness in each group—was used for pharmacoeconomic analysis.
Statistical Analyses
Categorical variables are reported as percentages, and normally distributed variables are reported as mean ± standard deviation. Analysis of variance and rank-sum tests were applied to compare continuous variables among the three groups. The Student–Newman–Keuls method or Dunnett’s t-test and Dunnett’s T3 were used for pairwise comparisons. The chi-square test was used for binary variables. The chi-square division method was adopted for pairwise comparison among the three groups, and the significance level was adjusted to α′,α′ = α/m, where m = k (k − 1)/2. The last observation carried forward (LOCF) approach was applied for missing values. P < 0.05 was considered statistically significant.
Results
Patient Characteristics
Of the 192 patients registered in this study, 183 [SEC (n = 87), ADA (n = 55), and MTX (n = 41)] had adequate baseline data (Table 1). The mean age was 37.73 years; the male-to-female ratio was approximately 2:1. The mean duration of psoriasis was 12.63 years, ranging from 2 months to 50 years. Approximately 20% of patients had a family history of psoriasis, and 21.86% had PsA at baseline.
However, certain differences in baseline characteristics were noted between patients in the SEC and ADA groups—including PsA comorbidity and previous biologics treatment—that may affect PASI 75 responsiveness. After correcting for these differences in the multiple logistic regression method used in the analysis (Table S1 in Supplementary Material), these factors were not significant. Instead, age and the drugs under consideration in our study (SEC or ADA) were significantly related to PASI 75 responsiveness.
Efficacy
Overall, the mean PASI score, BSA, and PGA score of the three groups showed a decline from baseline by week 4 and continued to decrease into week 12. In comparison, the SEC and ADA groups showed a greater decrease in average PASI score, BSA, and PGA score than the MTX group (Fig. S1 in Supplementary Material).
Responders whose PASI scores improved at weeks 4 and 12 by 50%, 75%, and 90% in each of the three treatment groups are shown in Fig. 1a–c. A higher percentage of responders was observed in patients who received SEC at week 4 and week 12, with fewer responders in patients treated with MTX. Further comparison of the number of patients who achieved PASI 75 between each of two groups at week 12 showed that differences were statistically significant (P1, P2, P3 < 0.01; P1 ADA versus SEC; P2 ADA versus MTX; P3 SEC versus MTX). Compared with the other two groups, there was a substantial PGA score improvement in the SEC group at week 12, with the highest percentage of patients achieving a PGA of 0 or 1—as shown in Fig. 1d. Clinical pictures for each group are shown in Fig. 2.
Improvement was statistically significant in the DLQI score from baseline to week 12 in both biologic groups, with DLQI score of 15.24 ± 5.71 to 5.91 ± 5.64 in the ADA group and 14.33 ± 6.33 to 2.32 ± 3.26 in the SEC group.
Safety and Pharmacoeconomic Analyses
ADA was associated with a significantly higher incidence of AEs than SEC (P = 0.03) (Table 2). AEs were reported by 31 (56.36%) and 39 (44.83%) patients in the ADA and SEC groups, respectively. However, no SAEs were observed in either of the two biologic groups in our study. The most common AE was upper respiratory infection in both groups. There was also a higher proportion of patients with injection site reactions in the ADA group (12.73%) than the SEC group (2.30%).
Based on actual drug prices during our study, the price of adalimumab was 1,290.00 Chinese yuan (CNY) each and secukinumab was 2,998.00 CNY each, the cost of SEC treatment per person over 12 weeks was 41,972.00 CNY, while it was 10,320.00 CNY in the ADA group. Therefore, the cost-effectiveness ratio in the SEC group was 46,331.83 CNY, compared with 17,580.92 CNY in the ADA group. Hence, we concluded that ADA was associated with lower cost-effectiveness ratio than SEC in the absence of health policy considerations. Pharmacoeconomic analysis should be reevaluated when drug prices and medical insurance policies change (Table S2 in Supplementary Material).
Discussion
In this study, we summarized the real-world efficacy and safety of biological agents for the treatment of psoriasis in a Chinese population involving 183 patients with psoriasis over 2 years. Concurrently, we used multiple logistic regression to exclude factors influencing PASI 75 responsiveness and calculated the relative cost-effectiveness of SEC and ADA.
The percentage of PASI 75 responders with PGA score of 0/1 was higher in the SEC group than in the ADA and MTX groups. Meanwhile, the incidence of AEs was lower in the SEC group than in the ADA group—consistent with findings reported from randomized controlled trials (RCTs), meta-analysis, and real-world studies [10, 11]. A previous real-world study in Taiwan in 2020 compared the efficacy of four biologics and found that the PASI 75 response was optimal in SEC (91.3%), followed by ustekinumab (79.6%), ADA (64.3%), and etanercept (50.0%) [12]. Simultaneously, our results showed that DLQI scores improved significantly with decreasing of PASI scores, in keeping with previous studies [13].
The most common AE in both groups was upper respiratory tract infections (23.48%), similar to the results in RCTs [14,15,16]. It is worth noting that a high proportion of patients in our study suffered pruritus (14.55% and 9.20%) and fatigue (9.09% and 10.34%) after ADA and SEC injections, respectively. Fatigue is a common symptom in many chronic inflammatory diseases and is regarded as a phenomenon associated with PsA [17]. A clinical trial reported the beneficial effects of the TNF-α inhibitor ADA against fatigue [18]. These findings suggest that it is crucial to assess and manage fatigue and pruritus in future studies. In addition, three patients (6.38%) reported AEs associated with weight gain after 1 month of ADA treatments, which is commonly encountered during treatment with TNF-α inhibitors owing to their effects on lipid metabolism [19]. However, the risk factors associated with changes in weight were diverse in previous studies [20,21,22,23,24]. Consequently, guidelines recommend that patients with moderate-to-severe psoriasis should be weighed annually [4].
From the perspective of health economics, previous studies between biologics and pharmaceutical economics focused mainly on cost–benefit analysis and paid more attention to improving patients’ quality of life. A study in the Netherlands showed that the use of different biologics influenced cost-effectiveness in terms of both costs and health effects [25]. Owing to differences in the reimbursement ratio of various types of medical insurance in China, we used the market price of biologics instead of the price paid for reimbursement. It is worth mentioning that, during the study period, ADA was included in the Chinese medical insurance catalogue while SEC was not. Therefore, our findings led us to conclude that, although SEC has a higher PASI 75 response rate with a lower incidence of AEs and risk of tuberculosis activation than ADA, the latter may be a better choice for patients with limited economic means. In comparison, SEC is a better option for patients with moderate-to-severe psoriasis who can afford its higher cost and desire better skin clearance as well as higher quality of life. Nevertheless, pharmacoeconomic analysis should be reevaluated when drug prices and medical insurance policies change, to examine whether the results remain consistent with the original ones. Furthermore, the results of pharmacoeconomic analysis regarding the cost-effectiveness, cost–benefit and cost–utility of the two biological agents must be confirmed by further research.
This study had certain limitations. First, the three groups were not randomized owing to complexities in clinical practice. Second, some patients received other treatments such as topical remedies that may have affected the results. Moreover, our study was limited by the relatively small sample size at a single location. Therefore, multicenter studies are necessary to obtain more information.
Conclusions
Consistent with previous clinical trials and real-world experiences, our study demonstrated that SEC and ADA were effective and safe for moderate-to-severe plaque psoriasis in Chinese patients. On the basis of drug prices during our study period and without taking into consideration access to health insurance, ADA was more cost-effective in real-world practice. Consequently, we recommend different therapeutic strategies for patients based on case-specific needs and access to financial resources.
References
National Psoraisis Foundation. https://www.psoriasis.org/psoriasis-statistics/. Accessed 1 October 2015.
Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):205–12.
Ding X, Wang T, Shen Y, et al. Prevalence of psoriasis in China: a population-based study in six cities. Eur J Dermatol. 2012;22(5):663–7.
Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD–NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073–113.
Papp K, Thaçi D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390(10089):40–9.
Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258–69.
Menter A, Strober BE, Kaplan DH, et al. Joint AAD–NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–72.
Smith CH, Yiu ZZN, Bale T, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2020: a rapid update. Br J Dermatol. 2020;183(4):628–37.
Committee on Psoriasis CSoD. Guideline for the diagnosis and treatment of psoriasis in China (2018 complete edition). Chin J Dermatol. 2019;52(10):667–710.
Gordon KB, Langley RG, Leonardi C, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. 2006;55(4):598–606.
Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106–15.
Chen YC, Huang YT, Yang CC, et al. Real-world efficacy of biological agents in moderate-to-severe plaque psoriasis: an analysis of 75 patients in Taiwan. PLoS ONE. 2020;15(12): e0244620.
Hjalte F, Carlsson KS, Schmitt-Egenolf M. Sustained Psoriasis Area and Severity Index, Dermatology Life Quality Index and EuroQol-5D response of biological treatment in psoriasis: 10 years of real-world data in the Swedish National Psoriasis Register. Br J Dermatol. 2018;178(1):245–52.
Wu NL, Hsu CJ, Sun FJ, Tsai TF. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: Subanalysis from ERASURE phase III study. J Dermatol. 2017;44(10):1129–37.
Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41(12):1039–46.
Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38.
Skoie IM, Ternowitz T, Jonsson G, Norheim K, Omdal R. Fatigue in psoriasis: a phenomenon to be explored. Br J Dermatol. 2015;172(5):1196–203.
Papp K, Crowley J, Ortonne JP, et al. Adalimumab for moderate to severe chronic plaque psoriasis: efficacy and safety of retreatment and disease recurrence following withdrawal from therapy. Br J Dermatol. 2011;164(2):434–41.
Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91.
Mahé E, Reguiai Z, Barthelemy H, et al. Evaluation of risk factors for body weight increment in psoriatic patients on infliximab: a multicentre, cross-sectional study. J Eur Acad Dermatol Venereol. 2014;28(2):151–9.
Briot K, Garnero P, Le Henanff A, Dougados M, Roux C. Body weight, body composition, and bone turnover changes in patients with spondyloarthropathy receiving anti-tumour necrosis factor alpha treatment. Ann Rheum Dis. 2005;64(8):1137–40.
Gisondi P, Cotena C, Tessari G, Girolomoni G. Anti-tumour necrosis factor-alpha therapy increases body weight in patients with chronic plaque psoriasis: a retrospective cohort study. J Eur Acad Dermatol Venereol. 2008;22(3):341–4.
Saraceno R, Schipani C, Mazzotta A, et al. Effect of anti-tumor necrosis factor-alpha therapies on body mass index in patients with psoriasis. Pharmacol Res. 2008;57(4):290–5.
Briot K, Gossec L, Kolta S, Dougados M, Roux C. Prospective assessment of body weight, body composition, and bone density changes in patients with spondyloarthropathy receiving anti-tumor necrosis factor-alpha treatment. J Rheumatol. 2008;35(5):855–61.
Klijn SL, van den Reek J, van de Wetering G, van der Kolk A, de Jong E, Kievit W. Biologic treatment sequences for plaque psoriasis: a cost-utility analysis based on 10 years of Dutch real-world evidence from BioCAPTURE. Br J Dermatol. 2018;178(5):1181–9.
Acknowledgements
We first want to give our sincere thanks to all the patients who participated in this study.
Funding
This study was supported in part by the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant. ZYJC21050), and the Key R&D project of the Science and Technology Department of Sichuan Province (project no. 2021YFG0306). The journal’s Rapid Service Fee was funded by the authors.
Medical Writing and Editorial Assistance
All authors contributed to the interpretation of the data and conception of the manuscript and critically revised and approved its content. Editorial assistance for the preparation of this article was provided by Wiley (http://wileyeditingservices.com), whose funding assistance was supported by the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant. ZYJC21050), and the Key R&D project of the Science and Technology Department of Sichuan Province (project no. 2021YFG0306).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Wei Li and Wei Yan contributed to the conception of the study and participated in constructive discussions regarding the manuscript; Gaojie Li, Yuanxia Gu, and Qin Zou conducted the clinical aspects of the study and wrote the manuscript; Yiyi Wang and Yue Xiao performed the data analysis; Dengmei Xia and Tongying Zhan evaluated PASI scores and recorded the occurrence of adverse events during the follow-up period; and Xingli Zhou and Qian Wang contributed to collecting laboratory results and recording data.
Disclosures
Gaojie Li was a postgraduate at West China Hospital of Sichuan University during the research and now works at Chengdu Second People's Hospital. Dengmei Xia is a Ph.D. student at the West China Hospital of Sichuan University and works at the Affiliated Hospital of Southwest Medical University. Tongying Zhan was a postgraduate at West China Hospital of Sichuan University during the research and now works at Chengdu Women’s and Children’s Central Hospital. All data and research results from this study were obtained on behalf of and belong to West China Hospital. Gaojie Li, Yuanxia Gu, Qin Zou, Yiyi Wang, Yue Xiao, Dengmei Xia, Tongying Zhan, Xingli Zhou, Qian Wang, Wei Yan, and Wei Li have no conflicts of interest to disclose.
Compliance with Ethics Guidelines
This study was approved by the ethics committee of West China Hospital of Sichuan University (approved in 2020, number 234) and conducted in accordance with the Declaration of Helsinki (2000 EDITION) and/or all applicable federal and local regulations. Prior to enrolment, each patient signed a written informed consent form.
Data Availability
All data from this study are available from the corresponding authors.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
13555_2022_787_MOESM1_ESM.pdf
Supplementary file1 SUPLEMENTARY MATERIAL Fig. S1 Changes in PASI score (a), BSA (b), and PGA score (c) of three groups from baseline to week 12. PASI psoriasis area and severity index, BSA body surface area, PGA physician global assessment, SEC secukinumab, ADA adalimumab, MTX methotrexate. Table S1 Multivariate logistic regression analysis of the PASI 75 response at week 12 in each biologic group. PASI 75 psoriasis area and severity index reduction of 75%, BMI body mass index, PASI psoriasis area and severity index, PsA psoriatic arthritis, SEC secukinumab, ADA adalimumab. Table S2 Pharmacoeconomic reevaluation at present. CNY Chinese yuan, SEC secukinumab, ADA adalimuma, C is the cost of biologics per person in the first three months, E is the percentage of PASI 75 responsiveness (PDF 176 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, G., Gu, Y., Zou, Q. et al. Efficacy, Safety, and Pharmacoeconomic Analysis of Adalimumab and Secukinumab for Moderate-to-Severe Plaque Psoriasis: A Single-Center, Real-World Study. Dermatol Ther (Heidelb) 12, 2105–2115 (2022). https://doi.org/10.1007/s13555-022-00787-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00787-x