Abstract
Introduction
Adherence to topical treatments for psoriasis is reported to be poor. One key contributing factor is the inconvenience associated with formulations that may be greasy, time consuming to apply, and slow to absorb. There is a paucity of patient-reported outcome measures that evaluate psoriasis patients’ perceptions of treatment convenience. The Psoriasis Treatment Convenience Scale (PTCS) was therefore developed and validated.
Methods
Following a literature review of issues relating to convenience of topical treatments, important items were identified and a draft version of the PTCS was developed and underwent content validity testing (n = 20). The revised scale was included in a clinical trial of topical therapy (n = 794; NCT03308799), and psychometric testing was performed.
Results
The final questionnaire included five core items and one overall satisfaction question. In psychometric testing, the scale demonstrated stability across trial population, and good validity, reliability, and sensitivity.
Conclusion
The PTCS is a new, reliable, sensitive, validated tool for the assessment of patient-reported treatment convenience. Use of the PTCS will facilitate evaluation of convenience as part of the clinical development of topical therapies, and thus may help to improve patient adherence and, therefore, treatment outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
While topical therapies for psoriasis are highly effective, adherence outside of clinical trials is low. One major reason for this is lack of convenience in current therapies. |
Before the tool described in this paper was created, there were no validated tools to measure patient-reported convenience of treatment. |
In this study, a tool to measure patient-reported convenience in topical psoriasis treatments was created and validated. |
What was learned from the study? |
The Psoriasis Treatment Convenience Scale (PTCS) was shown to be a valid, sensitive, and reliable tool to measure convenience. |
Future use of the PTCS can facilitate evaluation of convenience in the development of topical therapies, and thus may help the improvement of patient adherence and, therefore, support patient-centric treatment. |
Introduction
Topical therapies are highly effective in clinical trials for psoriasis [1, 2]. However, a significant barrier to the real-world effectiveness of such treatments is maintaining adequate adherence to the prescribed treatment regimen. Adherence to topical psoriasis treatment is poor, both short term and long term, with patient-reported adherence in the range of 51–90%, and objectively measured adherence even lower [3]. Adherence to topical therapies is generally worse than to other treatment modalities [4]. Adherence levels also diminish over time [5], with no medication taken on 37.4% of days in the first month, rising to 50.9% of days in the final month of a 12-month study in psoriasis [6]. Poor adherence in trials occurs despite participants’ awareness that their behavior is being monitored within a clinical trial. Adherence to topical treatment in the general, nontrial patient population is worse. Among those patients who do use their treatment, dosage is frequently lower than recommended, and can be as low as 35% of the full dose [7]. Increasing adherence can improve the effectiveness of psoriasis treatment [5].
There are many factors that contribute to poor adherence, such as perceived sub-optimal efficacy or safety [8], as well as the onerous and time-consuming nature of treatment administration [7, 8], poor cosmetic characteristic [7, 9], greasiness [10], and messiness of treatment [11]. Convenience of the treatment modality is, therefore, an important factor in optimizing adherence to therapy, treatment effectiveness, and patient well-being. However, while there are a number of questionnaires to evaluate the patients’ psoriasis disease intensity and quality of life, there is a paucity of validated tools with which to evaluate patient-reported convenience of treatments in psoriasis. To address this lack of appropriate measures, we developed and validated a new patient-reported outcome (PRO) instrument to assess convenience of topical psoriasis treatments. The validation of the Psoriasis Treatment Convenience Scale (PTCS) followed US Food and Drug Administration (FDA) guidance on PRO validation [12] and ISPOR guidelines for establishing content validity [13, 14]. The scale was included in a phase III trial, where a specific psychometric analysis plan was developed focusing on validity, reliability, and sensitivity.
Methods
Step 1: Literature Review and Draft Scale
A review of the literature was conducted to explore the problems associated with daily use of topical treatments in psoriasis and the availability of a validated questionnaire assessing these problems.
As no validated questionnaire was identified, a literature review was conducted to identify key themes relating to treatment convenience to construct a conceptual framework. Hereafter, a first draft convenience scale was developed. The structure of the draft scale was based on that of the widely used Dermatology Life Quality Index (DLQI) [15].
Step 2: Content Validity
To explore the content validity of the draft scale items, three focus groups were conducted (two in US English and one in Spanish-speaking US citizens). The focus groups included 20 patients: 18 with self-reported mild or moderate psoriasis, and 2 with self-reported severe psoriasis (age 27–77 years). After content validity testing, an updated scale was developed.
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All subjects were over the age of 18 years and provided informed consent to participate in this study. All data used in the current study follows General Data Protection Regulation (GDPR) guidelines. Additional ethical approval for the current study was not required by the Institutional Review Board (IRB) at Wake Forest School of Medicine, as it formed part of an already accepted phase III study and was a nonintervention, qualitative focus group study. The Institutional Review Board of Wake Forest School of Medicine deemed the study exempt from needing IRB approval.
Step 3: Psychometric Testing and Statistical Analysis
The updated scale was included in a phase III clinical trial measuring treatment convenience at week 1, week 4, and week 8 in a population of psoriasis patients treated with either calcipotriene (CAL)/betamethasone dipropionate (BDP) cream, cream vehicle, or CAL/BDP topical suspension (clinicaltrials.gov identifier: NCT03308799). Participants had a clinical diagnosis of mild/moderate plaque psoriasis on the trunk and/or limbs according to PGA, a mPASI score of at least 2 (treatment area of 2–30% of the body) of at least 6 months’ duration.
Validation was performed in a two-step process using blinded PTCS data (part I) and unblinded data (part II), to limit bias. In part I, the scale was tested for reliability across baseline characteristics and internal consistency. In part II, using unblinded data including treatment and clinical outcomes, the focus was primarily on sensitivity of the scale. Only key elements of the findings will be presented here.
Part I
Part 1 analyses were cross-sectionally performed by visit, with a focus on the week 1 measurement.
Descriptive statistics were used to evaluate the influence of baseline characteristics and demographics, as well as possible floor–ceiling effects. Internal consistency reliability of the PTCS was assessed by calculating the Cronbach’s alpha for the PTCS total score (Q1–Q5). Item–total score correlations were calculated to assess the homogeneity of the PTCS scores. Each item was also correlated with the PTCS total score but omitting that item. A value of ≥ 0.20 was set as a benchmark for internal consistency.
These analyses were supplemented with item response theory (IRT) analyses to identify potentially uninformative items and potential differential item functioning. The IRT analyses will not be described here.
Part II
Part II analysis was based on all data, including treatment group information, in order to consolidate the results obtained in part I. The dataset included all data included in part I, as well as the randomized treatment arm and treatment outcomes [PGA treatment success, subjective global assessment (SGA), and modified PASI and affected body surface area (BSA) at week 2, week 4, week 6, and week 8].
A random-effects ANOVA model based on weekly treatment as fixed effect and subject as random effect was used to model both PTCS total score and overall satisfaction score. The sensitivity and intersubject reliability were assessed. The responsiveness/sensitivity of the PTCS was evaluated with Cohen’s D effect size, and calculated for active treatment versus control treatment for week 1, week 4, and week 8 as the difference in mean normalized by the standard deviation. Effect size thresholds were: < 0.35 = small, 0.35– < 0.65 = medium, ≥ 0.65 = large.
Inter- and intrasubject reliability was estimated, and intraclass correlation coefficients were calculated as
Results
The item-tracking matrix (Table 1) shows the evolution of the PTCS questionnaire through the development of the scale. In brief, questions 1, 2, 3, and overall satisfaction were developed from themes identified in the literature. Question 5 was added following an internal evaluation. Questions 4 and 6 were added following patient input during content validity interviews. Questions 1–5 and the overall satisfaction score were slightly revised and refined during the process.
Step 1: Literature Review and Draft Scale
The literature review identified many studies reporting a large adherence problem [3, 6, 7, 13, 16, 17]. A theme that was repeatedly highlighted was the importance of patient perspectives on treatment convenience and practicality [7,8,9,10,11].
We did identify some questionnaires that included one question on convenience (e.g., DLQI item 10: “Over the last week, how much of a problem has the treatment for your skin been, for example by making your home messy, or by taking up time?” [15]), or that focused on treatment satisfaction [18]; however, no questionnaires that specifically measure the construct of convenience of topical treatment were found.
From the literature review, four key areas relating to patient convenience when using topical treatments were noted:
-
1.
The product was easy to apply to your skin.
-
2.
The product absorbed quickly into your skin.
-
3.
The product made your skin feel greasy.
-
4.
You would like to use this product again.
The themes were adapted to form the first draft questionnaire, consisting of four core items (as above, with the addition of a question relating to the time/disruption involved in using treatment) and a global impact question. Response options were on a scale from 1 to 10.
Step 2: Content Validity
Patients generally found the scale important and relevant, the wording easy to understand, and the response options acceptable for purpose (Table 1). Minor adjustments to phrasing were proposed, but no key changes were recommended to the scale items.
Participants felt that an additional item on itch would improve the scale as this was considered a specific issue in the patients’ daily lives. A question on itch was therefore added, but during further discussions with expert advisors, it was agreed that itch should be considered as a symptom of psoriasis and not a treatment convenience parameter. The item was therefore deleted to maintain the focus of the scale on convenience/adherence, and as itch was planned as a standalone question in the clinical trial.
Step 3: Psychometric Testing, Part I
A total of 794 patients were randomized in the clinical trial. The proportion of patients completing the questionnaire was 92.6% (735) at week 1, 88.7% (704) at week 4, and 85.3% (677) at week 8.
Distribution Properties
There were considerable ceiling effects for Q1 and Q5, with more than 50% of the answers in the highest category (10). Only minor distributional differences were observed based on baseline characteristics. As expected, the distributions at week 4 and week 8 showed no obvious differences from week 1.
Internal Consistency and Reliability
All items were positively correlated with the total score. A clear inter-item correlation was found between Q2 and Q4, while Q1 and Q5 showed a moderate correlation. The rest of the inter-item correlations were modest. The total score was positively correlated with the overall satisfaction score.
Cronbach’s alpha was estimated to be 0.61, below the predefined value of 0.70, indicating low internal consistency reliability.
The adjusted correlations between each item and the PTCS total score (omitting that item) were all above the predefined threshold for internal consistency of 0.20. Q2 and Q4 showed the highest correlations (close to 0.50), whereas the other items varied between 0.25 and 0.35.
Rasch Model for Polytomous Items
Comparison of the Rasch model and general graded IRT model allowing for different item slope parameters clearly resulted in a rejection of the Rasch model. However, the estimation of the general graded IRT model needed many iterations to converge, and the majority of estimated parameters had standard errors close to zero. This means that the results from the model should be treated with caution.
As the total number of possible answering patterns using the ten-level items is 100,000, the resulting outcome tables are very sparse. Consequently, the chi-square distribution assumption of the likelihood ratio test is questionable.
To check the construct validity using Rasch models, the items (Q1–Q5) were recategorized into three-level items (I1–I5), to try to achieve equal frequencies for each level. Repeating the analysis with the three-level items still led to a rejection of the Rasch model, and looking at the intraclass correlations of the general model, the slopes fell into two categories: those with a steep slope (I2 and I4), indicating a strong ability to discriminate the latent trait, and those with a moderate slope (I1, I3, and I5), indicating a less strong discriminatory capacity. The likelihood ratio test confirmed that it is reasonable to reduce the general model to a model with two different item slopes.
Absence of Differential Item Functioning
Using the ten-level items, the answer patterns for the different baseline groupings did not overlap, making these groups incomparable. Using the three-level items described in the previous section, none of the baseline groupings revealed differential item functioning.
Psychometric Testing: Part II
Sensitivity Analysis (Responsiveness)
At week 8, the observed effect sizes of the CAL/BDP cream versus CAL/BDP topical suspension were 4.1 for the PTCS total score and 0.8 for the overall satisfaction score, leading to moderate relative effect sizes (PTCS total score: 0.57; overall satisfaction: 0.36), as measured by Cohen’s D. The observed effect sizes of the CAL/BDP cream versus cream vehicle were 4.8 for the total score and 3.0 for the overall satisfaction score, leading to moderate relative effect sizes (PTCS total score: 0.67; overall satisfaction: 1.34), as measured by Cohen’s D.
Relative effect sizes of items Q2 and Q4 were most pronounced for MC2-01 cream versus active comparator, whereas for items Q2, Q3, and Q4, they were most pronounced for MC2-01 cream versus vehicle.
Intra- and Intersubject Reliability
For both the PTCS total score and the overall satisfaction, the between-subject variation accounted for more than 60% of the total variation. The variation between repeated measures within a subject accounted for less than 40%.
Discussion
Psoriasis is a chronic condition and patients are faced with the need for long-term treatment to help manage their symptoms [19]. Topical therapies can be highly effective in mild-to-moderate disease, but are frequently not used according to prescribing advice [3]. The importance of patient preference in the selection of topical treatment is highlighted by the recently published joint AAD-NPF guidelines of care for the management and care of psoriasis with topical therapy [20].
One reason for patients’ difficulties in maintaining treatment adherence is the inconvenience associated with the use of topical therapies that may be greasy, take time to absorb into the skin, or be difficult to apply. Despite the importance of these factors for patients’ daily lives, a validated scale with which to evaluate patient perceptions of these parameters has been lacking.
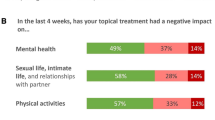
We developed and validated the new PTCS questionnaire to enable clinicians and researchers to take account of patient-reported convenience of, and satisfaction with, topical treatment. The PTCS scale is very simply constructed, with only two domains (Fig. 1). Overall, the patients found the items important and relevant, the wording easy to understand, and the response options acceptable for purpose. The psychometric testing showed a modest inter-item correlations, and a modest-to-good relative effect size, indicating that the scale has a high sensitivity to detect differences between topical psoriasis treatments in trial populations of this size. The reliability of both the PTCS total score and the overall satisfaction score demonstrated moderate-to-good reliability for repeated use of the scale.
In a phase III clinical trial enrolling 796 patients with mild-to-moderate plaque psoriasis, the mean PTCS at week 8 for a CAL/BDP cream was rated at 41.5 points out of 50 [21], and was evaluated overall to have superior PTCS compared with the CAL/BDP topical suspension, suggesting that the PTCS can differentiate between different topical formulations. For this particular comparison, the greatest difference was observed in PTCS questions related to the greasiness of the formulation.
Conclusions
Overall, the PTCS is a reliable, sensitive, and valid scale that reflects a specific and important characteristic of psoriasis treatment, namely treatment convenience. It will therefore be a vital tool in determining the relative benefits of new formulations, with different modes of action, on this parameter. It will also facilitate increased shared decision-making between doctors and patients. Shared decision-making, and a strong and cooperative physician–patient relationship, can increase patient adherence and treatment success, but they are dependent on clinicians understanding treatment preferences and satisfaction from the perspective of the psoriasis patient [22, 23]. Scales such as the PTCS may help identify treatment qualities that matter to the psoriasis patient and help increase patient adherence. The PTCS has only been tested in a specific patient population, and the true benefit of this tool will increase with evidence provided from other patient groups suffering from psoriasis.
Treatment convenience is not a direct indication of adherence. However, convenience is a key component of the patient experience of using treatment and is likely to influence patients’ willingness and ability to follow treatment recommendations.
References
Hendriks AGM, Keijsers RRMC, de Jong EMGJ, Seyger MMB, van de Kerkhof PCM. Efficacy and safety of combinations of first-line topical treatments in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(8):931–51.
Lebwohl M. Psoriasis. Ann Intern Med. 2018;168(7):Itc49-itc64.
Svendsen MT, Jeyabalan J, Andersen KE, Andersen F, Johannessen H. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28(5):374–83.
Saeki H, Imafuku S, Abe M, et al. Poor adherence to medication as assessed by the Morisky Medication Adherence Scale-8 and low satisfaction with treatment in 237 psoriasis patients. J Dermatol. 2015;42(4):367–72.
Carroll CL, Feldman SR, Camacho FT, Manuel JC, Balkrishnan R. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51(2):212–6.
Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176(3):759–64.
Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(Suppl 3):61–7.
Thorneloe RJ, Bundy C, Griffiths CEM, Ashcroft DM, Cordingley L. Nonadherence to psoriasis medication as an outcome of limited coping resources and conflicting goals: findings from a qualitative interview study with people with psoriasis. Br J Dermatol. 2017;176(3):667–76.
Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19(Suppl 3):2–6.
Svendsen MT, Andersen KE, Andersen F, Hansen J, Pottegård A, Johannessen H. Psoriasis patients’ experiences concerning medical adherence to treatment with topical corticosteroids. Psoriasis (Auckl). 2016;6:113–9.
Chan SA, Hussain F, Lawson LG, Ormerod AD. Factors affecting adherence to treatment of psoriasis: comparing biologic therapy to other modalities. J Dermatolog Treat. 2013;24(1):64–9.
U.S. Food and Drug Administration. Patient-reported outcome measures: use in medical product development to support labeling claims. U.S. Food and Drug Administration.
Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity–establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1–eliciting concepts for a new PRO instrument. Value Health. 2011;14(8):967–77.
Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity–establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2–assessing respondent understanding. Value Health. 2011;14(8):978–88.
Finlay AY, Khan GK. Dermatology life quality index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–6.
Belinchón I, Rivera R, Blanch C, Comellas M, Lizán L. Adherence, satisfaction and preferences for treatment in patients with psoriasis in the European Union: a systematic review of the literature. Patient Prefer Adherence. 2016;10:2357–67.
Svendsen MT, Andersen F, Hansen J, Johannessen H, Andersen KE. Medical adherence to topical corticosteroid preparations prescribed for psoriasis: a systematic review. J Dermatolog Treat. 2017;28(1):32–9.
Radtke MA, Spehr C, Reich K, Rustenbach SJ, Feuerhahn J, Augustin M. Treatment satisfaction in psoriasis: development and use of the psoSat patient questionnaire in a cross-sectional study. Dermatology (Basel). 2016;232(3):334–43.
Segaert S, Calzavara-Pinton P, de la Cueva P, et al. Long-term topical management of psoriasis: the road ahead. J Dermatolog Treat. 2021;84(2):432–70.
Elmets CA, Korman NJ, Prater EF, Hariharan, Menter A, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2020.
Stein Gold L, Green LJ, Dhawan S, Vestbjerg B, Praestegaard M, Selmer J. A Phase 3, randomized trial demonstrating the improved efficacy and patient acceptability of fixed dose calcipotriene and betamethasone dipropionate cream. J Drugs Dermatol. 2021;20(4):420–5.
Florek AG, Wang CJ, Armstrong AW. Treatment preferences and treatment satisfaction among psoriasis patients: a systematic review. Arch Dermatol Res. 2018;310(4):271–319.
Eicher L, Knop M, Aszodi N, Senner S, French LE, Wollenberg A. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease - strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33(12):2253–63.
Acknowledgements
We would like to thank the participants of this study for their help in validating the PTCS
Funding
The validation study, including the rapid service fee, was financially supported by MC2 Therapeutics.
Disclosures
Steve Feldman has received research, speaking and/or consulting support from Galderma, GSK/Stiefel, Almirall, Alvotech, Leo Pharma, BMS, Boehringer Ingelheim, Mylan, Amgen, Pfizer, Ortho Dermatology, Abbvie, Samsung, Janssen, Lilly, Menlo, Merck, Novartis, Regeneron, Sanofi, Novan, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Suncare Research, Informa, UpToDate and National Psoriasis Foundation. He is founder and majority owner of www.DrScore.com and a founder and part owner of Causa Research, a company dedicated to enhancing patients’ adherence to treatment. Arne Andreasen and Tove Holm-Larsen have received research and consulting support from MC2. Morten Præstegaard and Johan Selmer are both full time employeed at MC2 Therapeutics.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Feldman, Præstegaard, Selmer, and Holm-Larsen contributed to the study design and methods. Feldman contributed especially to the clinical design, Præstegaard and Selmer contributed especially to setup, Holm-Larsen contributed especially to qualitative interview and content validity. Andreasen contributed to the statistical analysis.
Compliance with Ethics Guidelines
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All subjects were above the age of 18 and provided informed consent to participate in this study. All data used in the current study follows GDPR guidelines. Additional ethical approval for the current study was not required by the IRB at Wake Forest School of Medicine, as it formed part of an already accepted phase III study and was a non-intervention, qualitative focus group study. The Institutional Review Board of Wake Forest School of Medicine deemed the study exempt from needing IRB approval.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author, Dr. Tove Holm-Larsen, on reasonable request and in accordance with GDPR legislation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Feldman, S.R., Præstegaard, M., Andreasen, A.H. et al. Validation of the Self-Reported Psoriasis Treatment Convenience Scale (PTCS). Dermatol Ther (Heidelb) 11, 2077–2088 (2021). https://doi.org/10.1007/s13555-021-00626-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-021-00626-5