Abstract
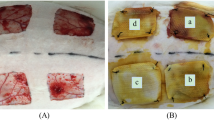
Skin damages are usual physical injuries and different studies have been done to improve wound healing. Hydrogel due to its properties like a moist environment and cooling wound site is a good option for wound treatment. In this study, we evaluated the consequence of using alginate/chitosan hydrogel contained various dosages of 4-Methylcatechol (0, 0.1, 1% (W/W)) on wound healing. After hydrogel fabrication, different tests like SEM, swelling, release, weight loss, and hemo- and cytocompatibility were done to characterize fabricated hydrogels. Finally, the rat model was used to assess Alginate/Chitosan hydrogel's therapeutic function containing 0.1 and 1% of 4-Methylcatechol. The pore size of hydrogel was between 24.5 ± 9 and 62.1 ± 11.63 µm and about 90% of hydrogel was lost after 14 days in the weight loss test. Blood compatibility and MTT assay showed that hydrogels were nontoxic and improved cell proliferation. In vivo test showed that Alginate/Chitosan/0.1%4-Methylcatechol improved wound healing and the results were significantly better than the gauze-treated wound. Our results showed dose depending effect of 4-Methylcatechol on wound healing. This study shows the treatment effect of 4-Methylcatechol on wound healing and the possibility of using it for treating skin injuries.







Similar content being viewed by others
References
Nyame TT, Chiang HA, Leavitt T, et al. Tissue-engineered skin substitutes. Plast Reconstr Surg. 2015;136(6):1379–88.
Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen. 1994;2(3):165–70.
Chaudhari AA, Vig K, Baganizi DR, et al. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: a review. Int J Mol Sci. 2016;17(12):1974.
Mogoşanu GD, Grumezescu AM. Natural and synthetic polymers for wounds and burns dressing. Int J Pharm. 2014;463(2):127–36.
Gonzalez ACDO, Costa TF, Andrade ZDA, et al. Wound healing-A literature review. An Bras Dermatol. 2016;91(5):614–20.
Talikowska M, Fu X, Lisak G. Application of conducting polymers to wound care and skin tissue engineering: a review. Biosensors and Bioelectronics. 2019.
Jaffe L, Wu SC. Dressings, topical therapy, and negative pressure wound therapy. Clin Podiatr Med Surg. 2019;36(3):397–411.
Dhivya S, Padma VV, Santhini E. Wound dressings–a review. BioMedicine. 2015;5(4).
O’brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14(3):88–95.
Carletti E, Motta A, Migliaresi C. Scaffolds for tissue engineering and 3D cell culture: 3D cell culture. Springer; 2011. p. 17–39.
Bendrea A-D, Cianga L, Cianga I. Progress in the field of conducting polymers for tissue engineering applications. J Biomater Appl. 2011;26(1):3–84.
Huynh CT, Lee DS. Controlling the properties of poly (amino ester urethane)–poly (ethylene glycol)–poly (amino ester urethane) triblock copolymer pH/temperature-sensitive hydrogel. Colloid Polym Sci. 2012;290(11):1077–86.
Jeong K-H, Park D, Lee Y-C. Polymer-based hydrogel scaffolds for skin tissue engineering applications: a mini-review. J Polym Res. 2017;24(7):112.
Bagher Z, Ehterami A, Safdel MH, et al. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. J Drug Deliv Sci Technol. 2020;55: 101379.
Pereira R, Carvalho A, Vaz DC, et al. Development of novel alginate based hydrogel films for wound healing applications. Int J Biol Macromol. 2013;52:221–30.
Thu H-E, Zulfakar MH, Ng S-F. Alginate based bilayer hydrocolloid films as potential slow-release modern wound dressing. Int J Pharm. 2012;434(1–2):375–83.
Karimi S, Bagher Z, Najmoddin N, et al. Alginate-magnetic short nanofibers 3D composite hydrogel enhances the encapsulated human olfactory mucosa stem cells bioactivity for potential nerve regeneration application. Int J Biol Macromol. 2021;167:796–806.
Pereira R, Mendes A, Bártolo P. Alginate/Aloe vera hydrogel films for biomedical applications. Procedia CIRP. 2013;5:210–5.
Ribeiro MP, Espiga A, Silva D, et al. Development of a new chitosan hydrogel for wound dressing. Wound Repair Regen. 2009;17(6):817–24.
Garakani SS, Khanmohammadi M, Atoufi Z, et al. Fabrication of chitosan/agarose scaffolds containing extracellular matrix for tissue engineering applications. Int J Biol Macromol. 2020;143:533–45.
Hilmi M, Bakar A, Halim AS, et al. Chitosan dermal substitute and chitosan skin substitute contribute to accelerated full-thickness wound healing in irradiated rats. BioMed Res Int. 2013;2013.
Garakani SS, Davachi SM, Bagher Z, et al. Fabrication of chitosan/polyvinylpyrrolidone hydrogel scaffolds containing PLGA microparticles loaded with dexamethasone for biomedical applications. Int J Biol Macromol. 2020;164:356–70.
Murakami K, Aoki H, Nakamura S, et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials. 2010;31(1):83–90.
Torelli-Souza RR, Cavalcante Bastos LA, Nunes HG, et al. Sustained release of an antitumoral drug from alginate-chitosan hydrogel beads and its potential use as colonic drug delivery. J Appl Polym Sci. 2012;126(S1):E409–18.
Applová L, Karlíčková J, Warncke P, et al. 4-methylcatechol, a flavonoid metabolite with potent antiplatelet effects. Mol Nutr Food Res. 2019;63(20):1900261.
Thiede H-M, Kehr W. 4-methylcatechol Derivatives and Uses Thereof. Google Patents; 2019.
Hsieh Y-L, Lin W-M, Lue J-H, et al. Effects of 4-methylcatechol on skin reinnervation: promotion of cutaneous nerve regeneration after crush injury. J Neuropathol Exp Neurol. 2009;68(12):1269–81.
Saita K, Ohi T, Hanaoka Y, et al. Effects of 4-methylcatechol, a stimulator of endogenous nerve growth factor synthesis, on experimental acrylamide-induced neuropathy in rats. Neurotoxicology. 1995;16(3):403–12.
Rahmati M, Ehterami A, Saberani R, et al. Improving sciatic nerve regeneration by using alginate/chitosan hydrogel containing berberine. Drug Deliv Transl Res. 2020:1–11.
Abbaszadeh-Goudarzi G, Haghi-Daredeh S, Ehterami A, et al. Evaluating effect of alginate/chitosan hydrogel containing 4-Methylcatechol on peripheral nerve regeneration in rat model. Int J Polym Mater Polym Biomater. 2020:1–10.
Ehterami A, Salehi M, Farzamfar S, et al. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J Drug Deliv Sci Technol. 2019.
Ehterami A, Salehi M, Farzamfar S, et al. In vitro and in vivo study of PCL/collagen wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats model. Int J Biol Macromol. 2018.
Salehi M, Vaez A, Naseri-Nosar M, et al. Naringin-loaded Poly (ε-caprolactone)/gelatin electrospun mat as a potential wound dressing: in vitro and in vivo evaluation. Fibers Polym. 2018;19(1):125–34.
Feng G, Nguyen TD, Yi X, et al. Evaluation of long-term inflammatory responses after implantation of a novel fully bioabsorbable scaffold composed of poly-l-lactic acid and amorphous calcium phosphate nanoparticles. J Nanomater. 2018;2018.
Liao J, Jia Y, Wang B, et al. Injectable hybrid poly (ε-caprolactone)-b-poly (ethylene glycol)-b-poly (ε-caprolactone) porous microspheres/alginate hydrogel cross-linked by calcium gluconate crystals deposited in the pores of microspheres improved skin wound healing. ACS Biomater Sci Eng. 2018;4(3):1029–36.
Varaprasad K, Jayaramudu T, Kanikireddy V, et al. Alginate-based composite materials for wound dressing application: a mini review. Carbohydr Polym. 2020:116025.
Das S, Pati F, Choi Y-J, et al. Bioprintable, cell-laden silk fibroin–gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015;11:233–46.
Karri VVSR, Kuppusamy G, Talluri SV, et al. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int J Biol Macromol. 2016;93:1519–29.
Mndlovu H, du Toit LC, Kumar P, et al. Development of a fluid-absorptive alginate-chitosan bioplatform for potential application as a wound dressing. Carbohyd Polym. 2019;222: 114988.
Venkatesan J, Lee J-Y, Kang DS, et al. Antimicrobial and anticancer activities of porous chitosan-alginate biosynthesized silver nanoparticles. Int J Biol Macromol. 2017;98:515–25.
Miguel SP, Moreira AF, Correia IJ. Chitosan based-asymmetric membranes for wound healing: a review. Int J Biol Macromol. 2019;127:460–75.
Hsieh Y-L, Kan H-W, Chiang H, et al. Distinct TrkA and Ret modulated negative and positive neuropathic behaviors in a mouse model of resiniferatoxin-induced small fiber neuropathy. Exp Neurol. 2018;300:87–99.
Thiede H-M, Kehr W. 4-methylcatechol Derivatives and Uses Thereof. Google Patents; 2016.
Furukawa Y, Urano T, Minamimura M, et al. 4-Methylcatechol-induced heme oxygenase-1 exerts a protective effect against oxidative stress in cultured neural stem/progenitor cells via PI3 kinase/Akt pathway. Biomed Res. 2010;31(1):45–52.
Hung S-Y, Liou H-C, Fu W-M. The mechanism of heme oxygenase-1 action involved in the enhancement of neurotrophic factor expression. Neuropharmacology. 2010;58(2):321–9.
Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12):1–17.
Yannas I, Lee E, Orgill DP, et al. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci. 1989;86(3):933–7.
O’Brien FJ, Harley BA, Yannas IV, et al. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26(4):433–41.
Ahmadi F, Oveisi Z, Samani SM, et al. Chitosan based hydrogels: characteristics and pharmaceutical applications. Res Pharm Sci. 2015;10(1):1.
Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: a review of patents and commercial products. Eur Polymer J. 2015;65:252–67.
Venkatesan J, Bhatnagar I, Kim S-K. Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Mar Drugs. 2014;12(1):300–16.
Prang P, Müller R, Eljaouhari A, et al. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27(19):3560–9.
Jagetia GC, Ravikiran P. Acceleration of wound repair and regeneration by Nigella sativa in the deep dermal excision wound of mice whole body exposed to different doses of γ-radiation. Am Res J Med Surg. 2015;1(3):1–17.
Senger DR, Cao S. Diabetic wound healing and activation of Nrf2 by herbal medicine. J Nat Sci. 2016;2(11).
Ehterami A, Salehi M, Farzamfar S, et al. A promising wound dressing based on alginate hydrogels containing vitamin D3 cross-linked by calcium carbonate/d-glucono-δ-lactone. Biomed Eng Lett. 2020:1–11.
Salehi M, Ehterami A, Farzamfar S, et al. Accelerating healing of excisional wound with alginate hydrogel containing naringenin in rat model. Drug Deliv Transl Res. 2020:1–12.
Acknowledgements
The present study was supported by Shahroud University of Medical Sciences, Shahroud, Iran (Grant No. 97151).
Funding
The present study was supported by Shahroud University of Medical Sciences, Shahroud, Iran (Grant No. 97151).
Author information
Authors and Affiliations
Contributions
All of the authors contributed to different parts of this paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval and consent to participate
Animal experiments were approved by the ethics committee of the Shahroud university of medical sciences (ethical code: IR.SHMU.REC.1397.167) and were carried out in accordance with the university’s guidelines.
Consent for publication
All of the authors confirm the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Majidi Ghatar, J., Ehterami, A., Nazarnezhad, S. et al. A novel hydrogel containing 4-methylcatechol for skin regeneration: in vitro and in vivo study. Biomed. Eng. Lett. 13, 429–439 (2023). https://doi.org/10.1007/s13534-023-00273-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-023-00273-z