Abstract
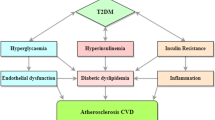
Diabetic dyslipidemia is characterised by low HDL-C and high triglyceride levels. Unlike the Caucasian population, though LDL-C levels are not very high, there is a preponderance of more atherogenic small, dense LDL particles among Indians. Furthermore, apo B levels are elevated. This, unique ‘atherogenic dyslipidemia’, is frequently encountered in South Asians with diabetes. People with type 2 diabetes are considered to be at high risk for vascular events. Hence, irrespective of other risk factors such as age, male gender, hypertension, family history, smoking, obesity, and polycystic ovary syndrome in women, they must be screened for dyslipidemia. Other major ASCVD risk factors include family history of hyperlipidemia, low levels of HDL-C, hypertriglyceridemia, and increased levels of total serum cholesterol level, non-HDL-C, LDL-C, apo B, Lp(a), triglyceride-rich remnants, and small, dense LDL-C. In patients with diabetes, dyslipidemia should be assessed at diagnosis and annually thereafter. In patients with type 1 diabetes, screening for dyslipidemia should be initiated from the age of 12 years. Periodical screening for dyslipidemia is recommended in overweight or obese children with a family history of type 2 diabetes, or those from a predisposed race/ethnicity like Asian, American Indian, etc. Both fasting and non-fasting lipid profiles are important for managing Indian patients with dyslipidemia. For routine screening, a fasting lipid profile is not mandatory; the decision to acquire fasting or non-fasting lipid values must be individually tailored. Apolipoprotein B level is considered an enhanced estimate of an individual’s exposure to atherosclerotic lipoproteins, and may be predominantly valuable for assessment of risk in individuals where LDL-C measurement underestimates this burden (those with diabetes mellitus, high triglycerides, obesity, or low LDL-C). The QRISK3 assessment tool algorithm calculates an individual’s risk of developing a heart attack or stroke over 10 years, and takes into account ethnicity as a risk factor. Considering the possible genetic influence of Indian ethnicity on CVD, the QRISK3 score exemplifies as the current most accurate CVD screening tool available for the Indian population.
Stratification of ASCVD risk in Indian diabetic patients:
• High risk: diabetes with 0–1 other major ASCVD risk factors and no evidence of target organ damage.
• Very high risk: diabetes with ≥2 other major ASCVD risk factors or evidence of target organ damage.
High-risk patients necessitate management comparable to that for secondary prevention of CVD. The most important step in defining treatment goals for dyslipidemia in diabetic patients is an extensive assessment of their cardiovascular risk, with LDL-C as the primary target, and non HDL-C, HDL-C, and apo B as secondary targets. A comprehensive strategy is essential in the management of dyslipidemia so as to regulate lipid levels and tackle related metabolic deviations and modifiable risk factors.
Essential considerations to improve lipid profile and glycemic control, and reduce CVD risk:
• Accomplish healthy weight and aerobic activity level,
• Implement an energy-restricted, well-balanced diet,
• No or at most moderate alcohol consumption, and
• Smoking (or any other tobacco use) cessation.
Medical nutrition therapy plays a central part in diabetes management; every individual with diabetes must be actively engaged in self-management, education, and treatment planning with their healthcare team, together with the collective development of an individualised eating plan. Statins are beneficial as a primary or secondary prevention strategy, to reduce the risk of cardiovascular events, in patients with ASCVD or multiple cardiovascular risk factors especially in those with diabetes. Unless contraindicated, first-line cholesterol-lowering therapy includes the use of moderate- to high-intensity statin. Ezetimibe, when combined with statins, provides additive and complementary therapeutic lipid effects, resulting in considerable reductions in LDL-C and significant achievement of target cholesterol levels. It also permits the use of lower dosage of statins without compromising efficacy, reducing the odds of dose-dependent statin adverse effects. Bempedoic acid seems to provide a safe and effective oral therapeutic option for lipid lowering in patients intolerant to statins. PCSK9 inhibitor therapy, in diabetes, induces analogous relative reductions in cardiovascular risk, and is recommended to further reduce LDL-C in patients aged 40–79 years with LDL-C ≥190 mg/dL, with ASCVD risk factors, or other significant additional-high risk markers (including diabetes) and LDL-C ≥100 mg/dL or non-HDL-C ≥130 mg/dL on maximally tolerated statin therapy and/or ezetimibe. Fenofibrate has shown to reduce CVD in diabetic patients with elevated triglycerides and low HDL-C levels. Saroglitazar has well-documented positive effects in the management of diabetic dyslipidemia; not only does it improve lipid parameters (triglycerides, apo B, non-HDL-C), it has a significant impact on glycemic parameters (HbA1c and fasting blood glucose) in dyslipidemic patients. It, hence, appears as a novel therapy for decreasing cardiovascular risk in patients with type 2 diabetes. Omega-3 fatty acids offer additional benefits when administered as an add-on to statins, and could be attributed to the lowering of detrimental chronic inflammatory markers in people with diabetes and high-risk cardiovascular patients. Icosapent ethyl may provide additional risk reduction benefit, beyond a statin, in individuals with ASCVD or diabetes and multiple risk factors and triglyceride ≥150 mg/dL. Considering the evidence in patients with diabetic dyslipidemia combined with the experience and consensus of the experts, we recommend a step-wise approach for the management for diabetic dyslipidemia in the Indian population (Table 7).


Similar content being viewed by others
References
Feingold KR, Grunfeld C. Diabetes and dyslipidemia. Endotext [Internet]. 2019 Jan 3.
Spratt KA. Managing diabetic dyslipidemia: aggressive approach. J Am Osteopathic Assoc. 2009;109(5_suppl_1):S2–7.
Gowtham K, Gandhe MB, Salwe KJ, Srinivasan AR. HDL/LDL ratio as a risk factor in type 2 diabetes mellitus. Adv Lab Med Int. 2012;2:9–18.
Chhatriwala MN, Patel MP, Patel DS, Shah HN. Relationship between dyslipidemia and glycemic status in type-2 diabetes mellitus. Natl J Lab Med. 2019;8(4):BO01–4.
Chandra KS, Bansal M, Nair T, Iyengar SS, Gupta R, Manchanda SC, et al. Consensus statement on management of dyslipidemia in Indian subjects. Indian Heart J. 2014;66(Suppl 3):S1.
Schofield JD, Liu Y, Rao-Balakrishna P, Malik RA, Soran H. Diabetes dyslipidemia. Diabetes Therapy. 2016;7(2):203–19.
Puri R, Mehta V, Iyengar SS, Narasingan SN, Duell PB, Sattur GB, et al. Lipid Association of India expert consensus statement on management of dyslipidemia in Indians 2020: Part III. J Assoc Phys India. 2020;68(11 [Special]):8–9.
Khan MA, Hashim MJ, King J, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes—global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020 Mar;10(1):107–11.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107843.
The World Bank [Website]. Diabetes prevalence. Accessed on 04th December 2019. Available from: https://data.worldbank.org/indicator/SH.STA.DIAB.ZS?end=2019&name_desc=false&start=2019&type=shaded&view=map&year=2019.
International Diabetes Federation. Updated on: 3rd March 2020. Cited on: 24th November 2020. Available from: https://idf.org/our-network/regions-members/south-east-asia/members/94-india.html.
Tandon N, Anjana RM, Mohan V, Kaur T, Afshin A, Ong K, et al. The increasing burden of diabetes and variations among the states of India: the Global Burden of Disease Study 1990–2016. Lancet Glob Health. 2018;6(12):e1352–62.
Parikh RM, Joshi SR, Menon PS, Shah NS. Prevalence and pattern of diabetic dyslipidemia in Indian type 2 diabetic patients. Diabetes Metab Syndr. 2010;4(1):10–2.
Joshi SR, Anjana RM, Deepa M, Pradeepa R, Bhansali A, et al. Prevalence of dyslipidemia in urban and rural India: the ICMR–INDIAB Study. PLoS ONE. 2014;9(5):e96808. https://doi.org/10.1371/journal.pone.0096808.
Mithal A, Majhi D, Shunmugavelu M, Talwarkar PG, Vasnawala H, Raza AS. Prevalence of dyslipidemia in adult Indian diabetic patients: a cross sectional study (SOLID). Indian J Endocrinol Metabol. 2014;18(5):642.
Dayakar E, Sree CS, Sanjay E. Study on the prevalence of dyslipidemia in type 2 diabetes mellitus. Int J Adv Med. 2019;6:786–9.
Bulut T, Demirel F, Metin A. The prevalence of dyslipidemia and associated factors in children and adolescents with type 1 diabetes. J Pediatr Endocrinol Metab. 2017;30(2):181–7.
Shah N, Khadilkar A, Gondhalekar K, Khadilkar V. Prevalence of dyslipidemia in Indian children with poorly controlled type 1 diabetes mellitus. Pediatr Diabetes. 2020;21(6):987–94.
Claypool KT, Chung MK, Deonarine A, Gregg EW, Patel CJ. Characteristics of undiagnosed diabetes in men and women under the age of 50 years in the Indian subcontinent: the National Family Health Survey (NFHS-4)/Demographic Health Survey 2015–2016. BMJ Open Diab Res Care. 2020;8(1):e000965.
Najafipour H, Shokoohi M, Yousefzadeh G, Azimzadeh BS, Kashanian GM, Bagheri MM, et al. Prevalence of dyslipidemia and its association with other coronary artery disease risk factors among urban population in Southeast of Iran: results of the Kerman coronary artery disease risk factors study (KERCADRS). J Diabetes Metab Disord. 2016;15(1):49.
Artha IM, Bhargah A, Dharmawan NK, Pande UW, Triyana KA, Mahariski PA, et al. High level of individual lipid profile and lipid ratio as a predictive marker of poor glycemic control in type-2 diabetes mellitus. Vasc Health Risk Manag. 2019;15:149.
Halcox J, Misra A. Type 2 diabetes mellitus, metabolic syndrome, and mixed dyslipidemia: how similar, how different, and how to treat? Metab Syndr Relat Disord. 2015;13(1):1–21.
Simha V. Management of hypertriglyceridemia. BMJ. 2020 Oct12;371:m3109.
Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5(3):150–9.
Panikar V. Chapter 101: mixed dyslipidemia. Medicine Update. 2008;18:764.
Kei A, Miltiadous G, Bairaktari E, Hadjivassiliou M, Cariolou M, Elisaf M. Dysbetalipoproteinemia: two cases report and a diagnostic algorithm. World J Clin Cases. 2015;3(4):371.
Varghese MJ. Familial hypercholesterolemia: a review. Ann Pediatr Cardiol. 2014;7(2):107.
Gaddi A, Cicero AF, Odoo FO. Practical guidelines for familial combined hyperlipidemia diagnosis: an up-date. Vasc Health Risk Manag. 2007;3(6):877.
Koopal C, Marais AD, Visseren FL. Familial dysbetalipoproteinemia: an underdiagnosed lipid disorder. Curr Opin Endocrinol Diabetes Obes. 2017;24(2):133–9.
Handelsman Y, Jellinger PS, Guerin CK, Bloomgarden ZT, Brinton EA, Budoff MJ, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the management of dyslipidemia and prevention of cardiovascular disease algorithm—2020 executive summary. Endocr Pract. 2020;26(10):1196–224.
Vodnala D, Rubenfire M, Brook RD. Secondary causes of dyslipidemia. Am J Cardiol. 2012;110(6):823–5.
Kolovou GD, Anagnostopoulou KK, Kostakou PM, Bilianou H, Mikhailidis DP. Primary and secondary hypertriglyceridaemia. Curr Drug Targets. 2009;10(4):336–43.
Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32(11):1345–61.
Musunuru K. Atherogenic dyslipidemia: cardiovascular risk and dietary intervention. Lipids. 2010;45(10):907–14.
Krishnamurthy V, Kerekoppa AR, Prabhakar B. Cross-sectional study of pattern of dyslipidemia and prevalence of atherogenic diabetic dyslipidemia in newly detected diabetic patients. Asian J Med Sci. 2019;10(6):45–9.
Gudbjartsson DF, Thorgeirsson G, Sulem P, Helgadottir A, Gylfason A, Saemundsdottir J, et al. Lipoprotein (a) concentration and risks of cardiovascular disease and diabetes. J Am Coll Cardiol. 2019;74(24):2982–94.
Vergès B. Lipid modification in type 2 diabetes: the role of LDL and HDL. Fundam Clin Pharmacol. 2009;23(6):681–5.
Femlak M, Gluba-Brzózka A, Ciałkowska-Rysz A, Rysz J. The role and function of HDL in patients with diabetes mellitus and the related cardiovascular risk. Lipids Health Dis. 2017;16(1):1–9.
Younis NN, Durrington PN. HDL functionality in diabetes mellitus: potential importance of glycation. Clin Lipidol. 2012;7(5):561–78.
Ganjali S, Dallinga-Thie GM, Simental-Mendía LE, Banach M, Pirro M, Sahebkar A. HDL functionality in type 1 diabetes. Atherosclerosis. 2017;267:99–109.
Farbstein D, Levy AP. HDL dysfunction in diabetes: causes and possible treatments. Expert Rev Cardiovasc Ther. 2012;10(3):353–61.
Lee JS, Chang PY, Zhang Y, Kizer JR, Best LG, Howard BV. Triglyceride and HDL-C dyslipidemia and risks of coronary heart disease and ischemic stroke by glycemic dysregulation status: the Strong Heart Study. Diabetes care. 2017;40(4):529–37.
Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Extreme lipoprotein (a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol. 2013;61(11):1146–56.
Willeit P, Kiechl S, Kronenberg F, Witztum JL, Santer P, Mayr M, et al. Discrimination and net reclassification of cardiovascular risk with lipoprotein (a): prospective 15-year outcomes in the Bruneck Study. J Am Coll Cardiol. 2014;64(9):851–60.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88.
Zhang HW, Zhao X, Guo YL, Gao Y, Zhu CG, Wu NQ, et al. Elevated lipoprotein (a) levels are associated with the presence and severity of coronary artery disease in patients with type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2018;28(10):980–6.
Singla S, Kaur K, Kaur G, Kaur H, Kaur J, Jaswal S. Lipoprotein (a) in type 2 diabetes mellitus: relation to LDL: HDL ratio and glycemic control. Int J Diabetes Dev Ctries. 2009;29(2):80.
Hermans MP, Ahn SA, Rousseau MF. The mixed benefit of low lipoprotein (a) in type 2 diabetes. Lipids Health Dis. 2017;16(1):171.
Zhang P, Gao J, Pu C, Zhang Y. Apolipoprotein status in type 2 diabetes mellitus and its complications. Mol Med Rep. 2017;16(6):9279–86.
Kanani FH, Alam JM. Apolipoprotein B in type 2 diabetics—a cross sectional study in a tertiary care set-up. J Pak Med Assoc. 2010;60(8):653.
Lee B, Pratumvinit B, Thongtang N. The role of apoB measurement in type 2 diabetic patients. Clin Lipidol. 2015;10(2):137–44.
Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(s2):1–87.
Chawla R, Madhu SV, Makkar BM, Ghosh S, Saboo B, Kalra S. RSSDI-ESI clinical practice recommendations for the management of type 2 diabetes mellitus 2020. Indian J Endocr Metab. 2020;24:1–122.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–143.
O’Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. 2007;100(5):899–904.
Langsted A, Nordestgaard BG. Nonfasting lipids, lipoproteins, and apolipoproteins in individuals with and without diabetes: 58 434 individuals from the Copenhagen General Population Study. Clin Chem. 2011;57(3):482–9.
Nakamura K, Miyoshi T, Yunoki K, Ito H. Postprandial hyperlipidemia as a potential residual risk factor. J Cardiol. 2016;67(4):335–9.
Anderson TJ, Mancini GJ, Genest J Jr, Grégoire J, Lonn EM, Hegele RA. The new dyslipidemia guidelines: what is the debate? Can J Cardiol. 2015;31(5):605–12.
de Vries MA. Novel pro-and anti-atherogenic effects of apolipoprotein B-containing lipoproteins: To feast or to fast? 2017.
Nordestgaard BG. A test in context: lipid profile, fasting versus nonfasting. J Am Coll Cardiol. 2017 Sep 18;70(13):1637–46.
Rahman F, Blumenthal RS, Jones SR, Martin SS, Gluckman TJ, Whelton SP. Fasting or non-fasting lipids for atherosclerotic cardiovascular disease risk assessment and treatment? Curr Atheroscler Rep. 2018;20(3):14.
Nordestgaard BG, Langsted A, Mora S, Kolovou G, Baum H, Bruckert E, et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points—a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J. 2016;37(25):1944–58.
Aldasouqi S, Sheikh A, Klosterman P, Kniestedt S, Schubert L, Danker R, et al. Hypoglycemia in patients with diabetes on antidiabetic medications who fast for laboratory tests. Diabetes Care. 2011;34(5):e52.
Mancini GJ, Hegele RA, Leiter LA. Dyslipidemia. Can J Diabetes. 2018;42:S178–85.
Sathiyakumar V, Park J, Golozar A, Lazo M, Quispe R, Guallar E, et al. Fasting versus nonfasting and low-density lipoprotein cholesterol accuracy. Circulation. 2018;137(1):10–9.
Stone NJ, Robinson JG, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, et al. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;63(25 Part B):2889–934.
Driver SL, Martin SS, Gluckman TJ, Clary JM, Blumenthal RS, Stone NJ. Fasting or nonfasting lipid measurements: it depends on the question. J Am Coll Cardiol. 2016;67(10):1227–34.
Yoshida H. Determination of fasting and non-fasting cholesterol levels of low-and high-density lipoproteins with homogenous assays: a promising reliable way to assessment of dyslipidemia. J Atheroscler Thromb. 2017;24(6):569–71.
Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118(10):993.
Doran B, Guo Y, Xu J, Weintraub H, Mora S, Maron DJ, et al. Prognostic value of fasting versus nonfasting low-density lipoprotein cholesterol levels on long-term mortality: insight from the National Health and Nutrition Examination Survey III (NHANES-III). Circulation. 2014;130(7):546–53.
Fatima S, Ijaz A, Sharif TB, Khan DA, Siddique A. Accuracy of non-fasting lipid profile for the assessment of lipoprotein coronary risk. J Coll Physicians Surg Pak. 2016;26:954–7.
Andrade C. Nonfasting lipid profile may suffice to manage dyslipidemia. Indian J Psychol Med. 2020;42(3):316–7.
Chamnan P, Simmons RK, Sharp SJ, Griffin SJ, Wareham NJ. Cardiovascular risk assessment scores for people with diabetes: a systematic review. Diabetologia. 2009;52(10):2001.
D’agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care. Circulation. 2008;117(6):743–53.
Woodward M, Brindle P, Tunstall-Pedoe H. Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC). Heart. 2007;93(2):172–6.
Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;23:357.
Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation. 2002;105(3):310–5.
Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611–9.
Ferrario M, Chiodini P, Chambless LE, Cesana G, Vanuzzo D, Panico S, et al. Prediction of coronary events in a low incidence population. Assessing accuracy of the CUORE Cohort Study prediction equation. Int J Epidemiol. 2005;34(2):413–21.
Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’agostino RB, Gibbons R, et al. ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;63(25 Part B):2935–59.
Hajifathalian K, Ueda P, Lu Y, Woodward M, Ahmadvand A, Aguilar-Salinas CA, et al. A novel risk score to predict cardiovascular disease risk in national populations (Globorisk): a pooled analysis of prospective cohorts and health examination surveys. Lancet Diabetes Endocrinol. 2015;3(5):339–55.
Jahangiry L, Farhangi MA, Rezaei F. Framingham risk score for estimation of 10-years of cardiovascular diseases risk in patients with metabolic syndrome. J Health Popul Nutr. 2017;36(1):1–6.
Stephens JW, Ambler G, Vallance P, Betteridge DJ, Humphries SE, Hurel SJ. Cardiovascular risk and diabetes. Are the methods of risk prediction satisfactory? Eur J Cardiovasc Prev Rehabil. 2004;11(6):521–8.
Stevens RJ, Kothari V, Adler AI, Stratton IM, Holman RR, United Kingdom Prospective Diabetes Study (UKPDS) Group. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin Sci. 2001;101(6):671–9.
QRISK®3 score [Website]. Version 2018.0. Last Updated on: 13th August 2018. Cited on: 19th April 2021. Available from: https://qrisk.org/three/index.php.
Ghosal S, Sinha B, Ved J, Biswas M. Quantitative measure of asymptomatic cardiovascular disease risk in type 2 diabetes: evidence from Indian outpatient setting. Indian Heart J. 2020;72(2):119–22.
Orringer CE, Blaha MJ, Blankstein R, Budoff MJ, Goldberg RB, Gill EA, et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021 Jan-Feb;15(1):33–60
Elkeles RS, Godsland IF, Feher MD, Rubens MB, Roughton M, Nugara F, et al. Coronary calcium measurement improves prediction of cardiovascular events in asymptomatic patients with type 2 diabetes: the PREDICT study. Eur Heart J. 2008;29(18):2244–51.
Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663–9.
Anand DV, Lim E, Hopkins D, Corder R, Shaw LJ, Sharp P, et al. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J. 2006;27:713–21.
Young LH, Frans JT, Chyun DA, Davey JA, Barrett EJ, Taillefer R, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301(15):1547–55.
Reiner Ž, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769–818.
Wu H, Shang H, Wu J. Effect of ezetimibe on glycemic control: a systematic review and meta-analysis of randomized controlled trials. Endocrine. 2018;60(2):229–39.
Kendall CW, Jenkins DJ. A dietary portfolio: maximal reduction of low-density lipoprotein cholesterol with diet. Curr Atheroscler Reports. 2004;6(6):492–8.
Jenkins DJ, Kendall CW, Faulkner DA, Nguyen T, Kemp T, Marchie A, et al. Assessment of the longer-term effects of a dietary portfolio of cholesterol-lowering foods in hypercholesterolemia. Am J Clin Nutr. 2006;83:582–91.
Kelley GA, Kelley KS. Impact of progressive resistance training on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Prev Med. 2009;48(1):9–19.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the study of obesity. Circulation. 2009;120(16):1640–5.
Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Look AHEAD Research Group, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–6.
American Diabetes Association. 5. Facilitating behavior change and well-being to improve health outcomes: standards of Medical Care in Diabetes 2021. Diabetes Care. 2021;44(Suppl. 1):S53–72.
Shantakumari N, Sequeira S. Effects of a yoga intervention on lipid profiles of diabetes patients with dyslipidemia. Indian Heart J. 2013;65(2):127–31.
Gordon L, McGrowder DA, Pena YT, Cabrera E, Lawrence-Wright M. Effect of exercise therapy on lipid parameters in patients with end-stage renal disease on hemodialysis. J Lab Physicians. 2012;4(1):17.
Nagarathna R, Tyagi R, Kaur G, Vendan V, Acharya IN, Anand A, et al. Efficacy of a validated yoga protocol on dyslipidemia in diabetes patients: NMB-2017 India trial. Medicines. 2019;6(4):100.
Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–92.
Boehm JK, Williams DR, Rimm EB, Ryff C, Kubzansky LD. Relation between optimism and lipids in midlife. Am J Cardiol. 2013;111(10):1425–31.
Evert AB, Dennison M, Gardner CD, Garvey WT, Lau KH, MacLeod J, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731–54.
Myers EF, Trostler N, Varsha V, Voet H. Insights from the Diabetes in India Nutrition Guidelines Study: adopting innovations using a knowledge transfer model. Top Clin Nutr. 2017;32(1):69.
Marincic PZ, Salazar MV, Hardin A, Scott S, Fan SX, Gaillard PR, et al. Diabetes self-management education and medical nutrition therapy: a multisite study documenting the efficacy of registered dietitian nutritionist interventions in the management of glycemic control and diabetic dyslipidemia through retrospective chart review. J Acad Nutr Diet. 2019;119(3):449–63.
Hegele RA, Gidding SS, Ginsberg HN, McPherson R, Raal FJ, Rader DJ, et al. Nonstatin low-density lipoprotein–lowering therapy and cardiovascular risk reduction—statement from ATVB council. Arterioscler, Thromb, Vasc Biol. 2015;35(11):2269–80.
Trialists CT. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371(9607):117–25.
Wang N, Fulcher J, Abeysuriya N, Park L, Kumar S, Di Tanna GL, et al. Intensive LDL cholesterol-lowering treatment beyond current recommendations for the prevention of major vascular events: a systematic review and meta-analysis of randomised trials including 327,037 participants. Lancet Diabetes Endocrinol. 2020;8(1):36–49.
Hadjiphilippou S, Ray KK. Cholesterol-lowering agents: statins—for everyone? Circ Res. 2019;124(3):354–63.
Khalil S, Khayyat S, Al-Khadra Y, Alraies MC. Should all diabetic patients take statin therapy regardless of serum cholesterol level? Expert Rev Cardiovasc Ther. 2019;17(4):237–9.
Naeem F, McKay G, Fisher M. Cardiovascular outcomes trials with statins in diabetes. Br J Diabetes. 2018;18(1):7–13.
Armani A, Toth PP. The CARDS trial: diabetic patients dealt a winning hand. Curr Atheroscler Rep. 2006;8(5):429–32.
Karlson BW, Barter PJ, Palmer MK, Lundman P, Nicholls SJ. Comparison of the effects of different statins and doses on lipid levels in patients with diabetes: results from VOYAGER. Nutr Metab Cardiovasc Dis. 2012;22(9):697–703.
Kawai Y, Sato-Ishida R, Motoyama A, Kajinami K. Place of pitavastatin in the statin armamentarium: promising evidence for a role in diabetes mellitus. Drug Des Devel Ther. 2011;5:283.
Barrios V, Escobar C. Clinical benefits of pitavastatin: focus on patients with diabetes or at risk of developing diabetes. Future Cardiol. 2016;12(4):449–66.
Martín-Timón I, Sevillano-Collantes C, García-Domínguez M, Marín-Peñalver JJ, Ugalde-Abiega B, del Cañizo-Gómez FJ. Update on the management of diabetic dyslipidaemia. EMJ Diabet. 2018;6(1):53–61.
Masana L. Pitavastatin in cardiometabolic disease: therapeutic profile. Cardiovasc Diabetol. 2013;12(1):1–8.
Mita T, Nakayama S, Abe H, Gosho M, Iida H, Hirose T, et al. Comparison of effects of pitavastatin and atorvastatin on glucose metabolism in type 2 diabetic patients with hypercholesterolemia. J Diabetes Investig. 2013;4(3):297–303.
Gumprecht J, Gosho M, Budinski D, Hounslow N. Comparative long-term efficacy and tolerability of pitavastatin 4 mg and atorvastatin 20–40 mg in patients with type 2 diabetes mellitus and combined (mixed) dyslipidaemia. Diabetes Obes Metab. 2011;13(11):1047–55.
Hoy SM. Pitavastatin: a review in hypercholesterolemia. Am J Cardiovasc Drugs. 2017;17(2):157–68.
Patil CY, Baig MS, Doifode SM. Assessing the efficacy and safety of pitavastatin compared to atorvastatin in dyslipidemic patients: a double blind randomized controlled trial. Int J Basic Clin Pharmacol. 2016;5:834–40.
Jayakumari C, Jabbar PK, Soumya S, Jayakumar RV, Das DV, Girivishnu G, et al. Lipid profile in Indian patients with type 2 diabetes: the scope for atherosclerotic cardiovascular disease risk reduction. Diabetes Spectr. 2020;33(4):299–306.
Stulc T, Ceška R, Gotto AM. Statin intolerance: the clinician’s perspective. Curr Atheroscler Rep. 2015;17(12):1–7.
Bitzur R, Cohen H, Kamari Y, Harats D. Intolerance to statins: mechanisms and management. Diabetes Care. 2013;36(Supplement 2):S325–30.
Alonso R, Cuevas A, Cafferata A. Diagnosis and management of statin intolerance. J Atheroscler Thromb. 2019 Mar 1;26(3):207–15.
Ferreira AM, da Silva PM. Defining the place of ezetimibe/atorvastatin in the management of hyperlipidemia. Am J Cardiovasc Drugs. 2017 Jun;17(3):169–81.
Barkas F, Elisaf M, Liberopoulos E, Klouras E, Liamis G, Rizos EC. Statin therapy with or without ezetimibe and the progression to diabetes. J Clin Lipidol. 2016 Mar 1;10(2):306–13.
Bohula EA, Giugliano RP, Cannon CP, Zhou J, Murphy SA, White JA, et al. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation. 2015;132(13):1224–33.
Giugliano RP, Cannon CP, Blazing MA, Nicolau JC, Corbalán R, Špinar J, et al. Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with versus without diabetes mellitus: results from IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation. 2018 Apr 10;137(15):1571–82.
Sakamoto K, Kawamura M, Watanabe T, Ashidate K, Kohro T, Tanaka A, et al. Effect of ezetimibe add-on therapy over 52 weeks extension analysis of prospective randomized trial (RESEARCH study) in type 2 diabetes subjects. Lipids Health Dis. 2017 Dec;16(1):1–9.
Lee J, Hwang YC, Lee WJ, Won JC, Song KH, Park CY, et al. Comparison of the efficacy and safety of rosuvastatin/ezetimibe combination therapy and rosuvastatin monotherapy on lipoprotein in patients with type 2 diabetes: multicenter randomized controlled study. Diabetes Ther. 2020 Feb;17:1–3.
Hong N, Lee YH, Tsujita K, Gonzalez JA, Kramer CM, Kovarnik T, et al. Comparison of the effects of ezetimibe-statin combination therapy on major adverse cardiovascular events in patients with and without diabetes: a meta-analysis. Endocrinol Metab. 2018 Jun;33(2):219.
Wang X, Zhang Y, Tan H, Wang P, Zha X, Chong W, et al. Efficacy and safety of bempedoic acid for prevention of cardiovascular events and diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2020 Dec;19(1):1–9.
Cicero AF, Fogacci F, Hernandez AV, Banach M. Lipid and Blood Pressure Meta-Analysis Collaboration (LBPMC) Group and the International Lipid Expert Panel (ILEP). Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: A systematic review and meta-analysis. PLoS Med. 2020;17(7):e1003121.
Laufs U, Banach M, Mancini GJ, Gaudet D, Bloedon LT, Sterling LR, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662.
Leiter L, Banach M, Catapano A, Duell P, Gotto A, Laufs U, et al. Bempedoic acid and glycemic control: a pooled analysis of 4 phase 3 clinical trials. J Clin Lipidol. 2020;14(4):577–9.
Rosenson RS, Hegele RA, Fazio S, Cannon CP. The evolving future of PCSK9 inhibitors. J Am Coll Cardiol. 2018;72(3):314–29.
Monami M, Sesti G, Mannucci E. PCSK9 inhibitor therapy: a systematic review and meta-analysis of metabolic and cardiovascular outcomes in patients with diabetes. Diabetes Obes Metab. 2019;21(4):903–8.
Pearson GJ, Thanassoulis G, Anderson TJ, Barry AR, Couture P, Dayan N, et al. 2021 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2021;37:1129–50.
Orringer CE, Jacobson TA, Saseen JJ, Brown AS, Gotto AM, Ross JL, et al. Update on the use of PCSK9 inhibitors in adults: recommendations from an Expert Panel of the National Lipid Association. J Clin Lipidol. 2017;11(4):880–90.
Handelsman Y, Lepor NE. PCSK9 inhibitors in lipid management of patients with diabetes mellitus and high cardiovascular risk: a review. J Am Heart Assoc. 2018;7(13):e008953.
Kosmas CE, Skavdis A, Sourlas A, Papakonstantinou EJ, Genao EP, Uceta RE, et al. Safety and tolerability of PCSK9 inhibitors: current insights. Clin Pharmacol. 2020;12:191.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Eng J Med. 2017;376(18):1713–22.
Sabatine MS, Leiter LA, Wiviott SD, Giugliano RP, Deedwania P, De Ferrari GM, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(12):941–50.
Ray KK, Leiter LA, Müller-Wieland D, Cariou B, Colhoun HM, Henry RR, et al. Alirocumab vs usual lipid-lowering care as add-on to statin therapy in individuals with type 2 diabetes and mixed dyslipidaemia: the ODYSSEY DM-DYSLIPIDEMIA randomized trial. Diabetes Obes Metab. 2018;20(6):1479–89.
Khan SU, Rahman H, Okunrintemi V, Riaz H, Khan MS, Sattur S, et al. Association of lowering low-density lipoprotein cholesterol with contemporary lipid-lowering therapies and risk of diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc. 2019;8(7):e011581.
Saha SA, Arora RR. Fibrates in the prevention of cardiovascular disease in patients with type 2 diabetes mellitus–a pooled meta-analysis of randomized placebo-controlled clinical trials. Int J Cardiol. 2010;141(2):157–66.
Hiukka A, Leinonen E, Jauhiainen M, Sundvall J, Ehnholm C, Keech AC, et al. Long-term effects of fenofibrate on VLDL and HDL subspecies in participants with type 2 diabetes mellitus. Diabetologia. 2007;50(10):2067–75.
Elam MB, Ginsberg HN, Lovato LC, Corson M, Largay J, Leiter LA, et al. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2(4):370–80.
d’Emden MC, Jenkins AJ, Li L, Zannino D, Mann KP, Best JD, et al. Favourable effects of fenofibrate on lipids and cardiovascular disease in women with type 2 diabetes: results from the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia. 2014;57(11):2296–303.
Tsunoda F, Asztalos IB, Horvath KV, Steiner G, Schaefer EJ, Asztalos BF. Fenofibrate, HDL, and cardiovascular disease in type-2 diabetes: the DAIS trial. Atherosclerosis. 2016;247:35–9.
Joshi SR. Saroglitazar for the treatment of dyslipidemia in diabetic patients. Expert Opin Pharmacother. 2015;16(4):597–606.
Sai VN, Pasula S, Sumathi S, Sreekanth M, Rao AS, Prasad BD. The clinical aspects of saroglitazar and its side effects. J Drug Deliv Ther. 2020;10(2):208–12.
Kaul U, Parmar D, Manjunath K, Shah M, Parmar K, Patil KP, et al. New dual peroxisome proliferator activated receptor agonist—saroglitazar in diabetic dyslipidemia and non-alcoholic fatty liver disease: integrated analysis of the real world evidence. Cardiovasc Diabetol. 2019;18(1):1–1.
Krishnappa M, Patil K, Parmar K, Trivedi P, Mody N, Shah C, et al. Effect of saroglitazar 2 mg and 4 mg on glycemic control, lipid profile and cardiovascular disease risk in patients with type 2 diabetes mellitus: a 56-week, randomized, double blind, phase 3 study (PRESS XII study). Cardiovasc Diabetol. 2020;19(1):1–3.
Backes J, Anzalone D, Hilleman D, Catini J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016;15(1):1–2.
Brinton EA, Ballantyne CM, Bays HE, Kastelein JJ, Braeckman RA, Soni PN. Effects of icosapent ethyl on lipid and inflammatory parameters in patients with diabetes mellitus-2, residual elevated triglycerides (200–500 mg/dL), and on statin therapy at LDL-C goal: the ANCHOR study. Cardiovasc Diabetol. 2013;12(1):1-0.
Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019;73(22):2791–802.
Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Eng J Med. 2019;380(1):11–22.
Natto ZS, Yaghmoor W, Alshaeri HK, Van Dyke TE. Omega-3 fatty acids effects on inflammatory biomarkers and lipid profiles among diabetic and cardiovascular disease patients: a systematic review and meta-analysis. Sci Rep. 2019;9(1):1-0.
Sunil B, Ashraf AP. Dyslipidemia in pediatric type 2 diabetes mellitus. Curr Diab Rep. 2020;20(10):1–9.
Zeitler P, Arslanian S, Fu J, Pinhas-Hamiel O, Reinehr T, Tandon N, et al. ISPAD clinical practice consensus guidelines 2018: type 2 diabetes mellitus in youth. Paedr diabetes. 2018;19:28–46.
American Diabetes Association. 13, Children and adolescents: standards of medical care in diabetes− 2020. Diabetes Care. 2020;43(Supplement 1):S163–82.
Barrett HL, Nitert MD, McIntyre HD, Callaway LK. Normalizing metabolism in diabetic pregnancy: is it time to target lipids? Diabetes Care. 2014;37(5):1484–93.
Wild R, Weedin EA, Wilson D. Dyslipidemia in pregnancy. Cardiology clinics. 2015;33(2):209–15.
Trialists CT. Articles Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407–15.
Gencer B, Marston NA, Im K, Cannon CP, Sever P, Keech A, et al. Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2020;396(10263):1637–43.
Ponce OJ, Larrea-Mantilla L, Hemmingsen B, Serrano V, Rodriguez-Gutierrez R, Spencer-Bonilla G, et al. Lipid-lowering agents in older individuals: a systematic review and meta-analysis of randomized clinical trials. J Clin Endocrinol Metab. 2019;104(5):1585–94.
Matsuzaka T, Shimano H. New perspective on type 2 diabetes, dyslipidemia and non-alcoholic fatty liver disease. J Diabetes Investig. 2020;11(3):532–4.
Marcum ZA, Griend JP, Linnebur SA. FDA drug safety communications: a narrative review and clinical considerations for older adults. Am J Geriatr Pharmacother. 2012;10(4):264–71.
Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126:1287–92.
Dongiovanni P, Petta S, Mannisto V, Mancina RM, Pipitone R, Karja V, et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63:705–12.
Mach F, Ray KK, Wiklund O, Corsini A, Catapano AL, Bruckert E, et al. Adverse effects of statin therapy: perception vs. the evidence–focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur Heart J. 2018;39(27):2526–39.
Bays HE, Cohen DE, Chalasani N, Harrison SA. An assessment by the Statin Liver Safety Task Force: 2014 update. J Clin Lipidol. 2014;8:S47–57.
Leoni S, Tovoli F, Napoli L, Serio I, Ferri S, Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: a systematic review with comparative analysis. World J Gastroenterol. 2018;24(30):3361–73.
Gawrieh S, Noureddin M, Loo NM, Mohseni R, Awasty VR, Cusi K, et al. A phase 2, prospective, multicenter, double-blind, randomized study of saroglitazar magnesium 1 mg, 2 mg or 4 mg versus placebo in patients with nonalcoholic fatty liver disease and/or nonalcoholic steatohepatitis (EVIDENCES IV). Hepatology. 2019;70(6):1484A–5A.
Goyal O, Nohria S, Goyal P, Kaur J, Sharma S, Sood A, et al. Saroglitazar in patients with non-alcoholic fatty liver disease and diabetic dyslipidemia: a prospective, observational, real world study. Scientific Reports. 2020;10(1):1–9.
Teramoto T. Pitavastatin: clinical effects from the LIVES Study. Atheroscler Suppl. 2011;12(3):285–8.
Wu Y, Wang Y, An C, Dong Z, Liu H, Zhang Y, et al. Effects of rosuvastatin and atorvastatin on renal function–meta-analysis. Circulation J. 2012;76(5):1259–66.
Rangel ÉB, de Sá JR, Melaragno CS, Gonzalez AM, Linhares MM, Salzedas A, et al. Kidney transplant in diabetic patients: modalities, indications and results. Diabetol Metab Syndr. 2009;1(1):1–7.
Breda A, Budde K, Figueiredo A, García EL, Olsburgh J, Regele H, et al. EAU guidelines on renal transplant – update 2021. Edn. presented at the EAU Annual Congress Milan Italy 2021. ISBN 978-94-92671-13-4.
Scicchitano P, Milo M, Mallamaci R, De Palo M, Caldarola P, Massari F, et al. Inclisiran in lipid management: a literature overview and future perspectives. Biomed Pharmacother. 2021;143:112227.
Wright RS, Collins MG, Stoekenbroek RM, Robson R, Wijngaard PL, Landmesser U, et al. Effects of renal impairment on the pharmacokinetics, efficacy, and safety of inclisiran: an analysis of the ORION-7 and ORION-1 studies. InMayo Clinic Proceedings. 2020;95(1):77–89.
Leiter LA, Teoh H, Kallend D, Wright RS, Landmesser U, Wijngaard PL, et al. Inclisiran lowers LDL-C and PCSK9 irrespective of diabetes status: the ORION-1 randomized clinical trial. Diabetes Care. 2019;42(1):173–6.
Acknowledgment
We acknowledge the assistance provided by consultant medical writers, Dr. Aafreen Saiyed and Dr. Suraj Ghag, in the preparation of these Guidelines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saboo, B., Agarwal, S., Makkar, B.M. et al. RSSDI consensus recommendations for dyslipidemia management in diabetes mellitus. Int J Diabetes Dev Ctries 42, 3–28 (2022). https://doi.org/10.1007/s13410-022-01063-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-022-01063-6