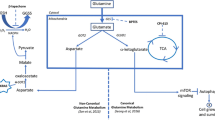
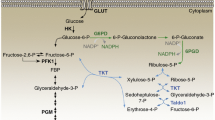
Abstract
Background
Metabolic changes have been recognized as an important hallmark of cancer cells. Cancer cells can promote their own growth and proliferation through metabolic reprogramming. Particularly, serine metabolism has frequently been reported to be dysregulated in tumor cells. 3-Phosphoglycerate dehydrogenase (PHGDH) catalyzes the first step in the serine biosynthesis pathway and acts as a rate-limiting enzyme involved in metabolic reprogramming. PHGDH upregulation has been observed in many tumor types, and inhibition of PHGDH expression has been reported to inhibit the proliferation of PHGDH-overexpressing tumor cells, indicating that it may be utilized as a target for cancer treatment. Recently identified inhibitors targeting PHGDH have already shown effectiveness. A further in-depth analysis and concomitant development of PHGDH inhibitors will be of great value for the treatment of cancer.
Conclusions
In this review we describe in detail the role of PHGDH in various cancers and inhibitors that have recently been identified to highlight progression in cancer treatment. We also discuss the development of new drugs and treatment modalities based on PHGDH targets. Overexpression of PHGDH has been observed in melanoma, breast cancer, nasopharyngeal carcinoma, parathyroid adenoma, glioma, cervical cancer and others. PHGDH may serve as a molecular biomarker for the diagnosis, prognosis and treatment of these cancers. The design and development of novel PHGDH inhibitors may have broad implications for cancer treatment. Therapeutic strategies of PHGDH inhibitors in combination with traditional chemotherapeutic drugs may provide new perspectives for precision medicine and effective personalized treatment for cancer patients.
Graphical abstract





Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
G.A. Barker, J.C. Ellory, The identification of neutral amino acid transport systems. Exp. Physiol. 75, 3–26 (1990)
M. Palacín, R. Estévez, J. Bertran, A. Zorzano, Molecular biology of mammalian plasma membrane amino acid transporters. Physiol. Rev. 78, 969–1054 (1998)
C.F. Labuschagne, N.J. van den Broek, G.M. Mackay, K.H. Vousden, O.D. Maddocks, Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 7(4), 1248–1258 (2014)
K.R. Mattaini, M.R. Sullivan, M.G. Vander, Heiden, The importance of serine metabolism in cancer. J. Cell Biol. 214, 249–257 (2016)
M.R. Sullivan, K.R. Mattaini, E.A. Dennstedt, A.A. Nguyen, S. Sivanand, M.F. Reilly, K. Meeth, A. Muir, A.M. Darnell, M.W. Bosenberg, C.A. Lewis, M.G. Vander Heiden, Increased serine synthesis provides an advantage for tumors arising in tissues where serine levels are limiting. Cell Metab. 29, 1410–1421 (2019)
C. Frezza, Cancer metabolism: addicted to serine. Nat. Chem. Biol. 12, 389–390 (2016)
K. Snell, Y. Natsumeda, G. Weber, The modulation of serine metabolism in hepatoma 3924A during different phases of cellular proliferation in culture. Biochem. J. 245, 609–612 (1987)
A.R. Mullen, R.J. DeBerardinis, Genetically-defined metabolic reprogramming in cancer. Trends Endocrinol. Metab. 23(11), 552–559 (2012)
E. Gottlieb, I.P. Tomlinson, Mitochondrial tumour suppressors: a genetic and biochemical update. Nat. Rev. Cancer 5(11), 857–866 (2005)
S.G. Dann, R.T. Abraham, Serine biosynthesis: fuel for the melanoma cell growth engine. Pigment Cell Melanoma Res. 24, 875–877 (2011)
E. Mullarky, L.L. Lairson, L.C. Cantley, C.A. Lyssiotis, A novel small-molecule inhibitor of 3-phosphoglycerate dehydrogenase. Mol. Cell. Oncol. 3, e1164280 (2016)
J. Luo, Cancer’s sweet tooth for serine. Breast Cancer Res. 13, 317 (2011)
E. Mullarky, K.R. Mattaini, M.G. Vander Heiden, L.C. Cantley, J.W. Locasale, PHGDH amplification and altered glucose metabolism in human melanoma. Pigment Cell Melanoma Res. 24, 1112–1115 (2011)
I. Kraoua, E. Wiame, L. Kraoua, F. Nasrallah, H. Benrhouma, A. Rouissi, I. Turki, H. Chaabouni, G. Briand, N. Kaabachi, E. Van Schaftingen, N. Gouider-Khouja, 3-Phosphoglycerate dehydrogenase deficiency: description of two new cases in Tunisia and review of the literature. Neuropediatrics 44(5), 281–285 (2013)
G.A. Grant, D-3-phosphoglycerate dehydrogenase. Front. Mol. Biosci. 5, 110 (2018)
A.M. Li, J.B. Ye, The PHGDH enigma: do cancer cells only need serine or also a redox modulator? Cancer Lett. 476, 97–105 (2020)
J.E. Unterlass, N.J. Curtin, Warburg and Krebs and related effects in cancer. Expert Rev. Mol. Med. 21, e4 (2019)
R.A. Cairns, I.S. Harris, T.W. Mak, Regulation of cancer cell metabolism. Nat. Rev. Cancer 11(2), 85–95 (2011)
O. Warburg, Über den stoffwechsel der carcinom-zelle. J. Mol. Med. 4(12), 534–536 (1925)
O. Warburg, On the origin of cancer cells. Science 123, 309–314 (1956)
O. Warburg, K. Posener, E. Negelein, Ueber den stoffwechsel der tumoren. Biochem. Z. 152, 319–344 (1924)
S.Y. Lunt, M.G. Vander Heiden, Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 (2011)
I. Amelio, F. Cutruzzolá, A. Antonov, M. Agostini, G. Melino, Serine and glycine metabolism in cancer. Trends Biochem. Sci. 39, 191–198 (2014)
J.W. Locasale, Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 (2013)
P.M. Tedeschi, E.K. Markert, M. Gounder, H. Lin, D. Dvorzhinski, S.C. Dolfi, L.L. Chan, J. Qiu, R.S. DiPaola, K.M. Hirshfield, L.G. Boros, J.R. Bertino, Z.N. Oltvai, A. Vazquez, Contribution of serine, folate and glycine metabolism to the ATP, NADPH and purine requirements of cancer cells. Cell Death Dis. 4, e877 (2013)
R.J. DeBerardinis, T. Cheng, Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29(3), 313–324 (2010)
R.J. Shaw, Glucose metabolism and cancer. Curr. Opin. Cell Biol. 18(6), 598–608 (2006)
T. Soga, Cancer metabolism: key players in metabolic reprogramming. Cancer Sci. 104, 275–281 (2013)
O. Kuge, K. Hasegawa, K. Saito, M. Nishijima, Control of phosphatidylserine biosynthesis through phosphatidylserine-mediated inhibition of phosphatidylserine synthase I in Chinese hamster ovary cells. Proc. Natl. Acad. Sci. U. S. A. 95, 4199–4203 (1998)
T.J. de Koning, K. Snell, M. Duran, R. Berger, B.T. Poll-The, R. Surtees, L-serine in disease and development. Biochem. J. 371, 653–661 (2003)
A.H. Futerman, H. Riezman, The ins and outs of sphingolipid synthesis. Trends Cell Biol. 15, 312–318 (2005)
M. Yang, K.H. Vousden, Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 16(10), 650–662 (2016)
J.S. Metcalf, R.A. Dunlop, J.T. Powell, S.A. Banack, P.A. Cox, L-serine: a naturally-occurring amino acid with therapeutic potential. Neurotox. Res. 33, 213–221 (2018)
K. Okabe, I. Usui, K. Yaku, Y. Hirabayashi, K. Tobe, T. Nakagawa, Deletion of PHGDH in adipocytes improves glucose intolerance in diet-induced obese mice. Biochem. Biophys. Res. Commun. 504, 309–314 (2018)
S. Vandekeere, C. Dubois, J. Kalucka, M.R. Sullivan, M. García-Caballero, J. Goveia, R. Chen, F.F. Diehl, L. Bar-Lev, J. Souffreau, A. Pircher, S. Kumar, S. Vinckier, Y. Hirabayashi, S. Furuya, L. Schoonjans, G. Eelen, B. Ghesquière, E. Keshet, X. Li, M.G. Vander Heiden, M. Dewerchin, P. Carmeliet, Serine synthesis via PHGDH is essential for heme production in endothelial cells. Cell Metab. 28, 573–587 (2018)
A. Schulze, A.L. Harris, How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 491, 364–373 (2012)
E. Piskounova, M. Agathocleous, M.M. Murphy, Z. Hu, S.E. Huddlestun, Z. Zhao, A.M. Leitch, T.M. Johnson, R.J. DeBerardinis, S.J. Morrison, Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191 (2015)
E. Mullarky, L.C. Cantley, Diverting glycolysis to combat oxidative stress. (Springer, Tokyo, 2015), pp. 3–23
J. Ye, J. Fan, S. Venneti, Y.W. Wan, B.R. Pawel, J. Zhang, L.W. Finley, C. Lu, T. Lindsten, J.R. Cross, G. Qing, Z. Liu, M.C. Simon, J.D. Rabinowitz, C.B. Thompson, Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 4, 1406–1417 (2014)
J.W. Locasale, A.R. Grassian, T. Melman, C.A. Lyssiotis, K.R. Mattaini, A.J. Bass, G. Heffron, C.M. Metallo, T. Muranen, H. Sharfi, A.T. Sasaki, D. Anastasiou, E. Mullarky, N.I. Vokes, M. Sasaki, R. Beroukhim, G. Stephanopoulos, A.H. Ligon, M. Meyerson, A.L. Richardson, L. Chin, G. Wagner, J.M. Asara, J.S. Brugge, L.C. Cantley, M.G. Vander Heiden, Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet. 43, 869–874 (2011)
R. Possemato, K.M. Marks, Y.D. Shaul, M.E. Pacold, D. Kim, K. Birsoy, S. Sethumadhavan, H.K. Woo, H.G. Jang, A.K. Jha, W.W. Chen, F.G. Barrett, N. Stransky, Z.Y. Tsun, G.S. Cowley, J. Barretina, N.Y. Kalaany, P.P. Hsu, K. Ottina, A.M. Chan, B. Yuan, L.A. Garraway, D.E. Root, M. Mino-Kenudson, E.F. Brachtel, E.M. Driggers, D.M. Sabatini, Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350 (2011)
D. Samanta, Y. Park, S.A. Andrabi, L.M. Shelton, D.M. Gilkes, G.L. Semenza, PHGDH expression is required for mitochondrial redox homeostasis, breast cancer stem cell maintenance, and lung metastasis. Cancer Res. 76, 4430–4442 (2016)
S. Pollari, S.M. Käkönen, H. Edgren, M. Wolf, P. Kohonen, H. Sara, T. Guise, M. Nees, O. Kallioniemi, Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res. Treat. 125, 421–430 (2011)
G.M. DeNicola, P.H. Chen, E. Mullarky, J.A. Sudderth, Z. Hu, D. Wu, H. Tang, Y. Xie, J.M. Asara, K.E. Huffman, I.I. Wistuba, J.D. Minna, R.J. DeBerardinis, L.C. Cantley, Erratum: NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nat. Genet. 48, 473 (2016)
S. Ravez, Q. Spillier, R. Marteau, O. Feron, R. Frédérick, Challenges and opportunities in the development of serine synthetic pathway inhibitors for cancer therapy. J. Med. Chem. 60, 1227–1237 (2017)
K. Snell, Enzymes of serine metabolism in normal, developing and neoplastic rat tissues. Adv. Enzyme Regul. 22, 325–400 (1984)
K. Snell, Enzymes of serine metabolism in normal and neoplastic rat tissues. Biochim. Biophys. Acta 843, 276–281 (1985)
K. Snell, The duality of pathways for serine biosynthesis is a fallacy. Trends Biochem. Sci. 11, 241–243 (1986)
D.A. Walsh, H.J. Sallach, Comparative studies on the pathways for serine biosynthesis in animal tissues. J. Biol. Chem. 241, 4068–4076 (1966)
G.P. Cheung, J. Cotropia, H.J. Sallach, The effects of dietary protein on the hepatic enzymes of serine metabolism in the rabbit. Arch. Biochem. Biophys. 129, 672–682 (1969)
E.V. Rowsell, K. Snell, J.A. Carnie, A.H. Al-Tai, Liver-L-alanine-glyoxylate and L-serine-pyruvate aminotransferase activities: an apparent association with gluconeogenesis. Biochem. J. 115, 1071–1073 (1969)
P. Stover, V. Schirch, Serine hydroxymethyltransferase catalyzes the hydrolysis of 5,10-methenyltetrahydrofolate to 5-formyltetrahydrofolate. J. Biol. Chem. 265, 14227–14233 (1990)
A.S. Tibbetts, D.R. Appling, R. Appling Dean, Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu. Rev. Nutr. 30, 57–81 (2010)
M.G. Vander Heiden, J.W. Locasale, K.D. Swanson, H. Sharfi, G.J. Heffron, D. Amador-Noguez, H.R. Christofk, G. Wagner, J.D. Rabinowitz, J.M. Asara, L.C. Cantley, Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science 329, 1492–1499 (2010)
J.W. Locasale, L.C. Cantley, Altered metabolism in cancer. BMC Biol. 8, 88 (2010)
L. Sun, L. Song, Q. Wan, G. Wu, X. Li, Y. Wang, J. Wang, Z. Liu, X. Zhong, X. He, S. Shen, X. Pan, A. Li, Y. Wang, P. Gao, H. Tang, H. Zhang, cMyc-mediated activation of serine biosynthesis pathway is critical for cancer progression under nutrient deprivation conditions. Cell Res. 25(4), 429–444 (2015)
G.A. Grant, A new family of 2-hydroxyacid dehydrogenases. Biochem. Biophys. Res. Commun. 165, 1371–1374 (1989)
C. Vinals, E. Depiereux, E. Feytmans, Prediction of structurally conserved regions of D-specific hydroxy acid dehydrogenases by multiple alignment with formate dehydrogenase. Biochem. Biophys. Res. Commun. 192, 182–188 (1993)
D.M. Greenberg, A. Ichihara, Further studies on the pathway of serine formation from carbohydrate. J. Biol. Chem. 224(1), 331–340 (1957)
J.D. Goldberg, T. Yoshida, P. Brick, Crystal structure of a NAD-dependent D-glycerate dehydrogenase at 2.4 Å resolution. J. Mol. Biol. 236, 1123–1140 (1994)
V.S. Lamzin, Z. Dauter, V.O. Popov, E.H. Harutyunyan, K.S. Wilson, High resolution structures of holo and apo formate dehydrogenase. J. Mol. Biol. 236, 759–785 (1994)
A. Ichihara, D.M. Greenberg, Studies on the purification and properties of D-glyceric acid kinase of liver. J. Biol. Chem. 225, 949–958 (1957)
J.E. Willis, H.J. Sallach, The occurrence of D-3-phosphoglycerate dehydrogenase in animal tissue. Biochim. Biophys. Acta 81(1), 39–54 (1964)
M.O. Kinoshita, Y. Shinoda, K. Sakai, T. Hashikawa, M. Watanabe, T. Machida, Y. Hirabayashi, S. Furuya, Selective upregulation of 3-phosphoglycerate dehydrogenase (PHGDH) expression in adult subventricular zone neurogenic niche. Neurosci. Lett. 453, 21–26 (2009)
L.W. Klomp, T.J. de Koning, H.E. Malingré, E.A. van Beurden, M. Brink, F.L. Opdam, M. Duran, J. Jaeken, M. Pineda, L. Van Maldergem, B.T. Poll-The, I.E. van den Berg, R. Berger, Molecular characterization of 3-phosphoglycerate dehydrogenase deficiency-a neurometabolic disorder associated with reduced L-serine biosynthesis. Am. J. Hum. Genet. 67, 1389–1399 (2000)
K. Yoshida, S. Furuya, S. Osuka, J. Mitoma, Y. Shinoda, M. Watanabe, N. Azuma, H. Tanaka, T. Hashikawa, S. Itohara, Y. Hirabayashi, Targeted disruption of the mouse 3-phosphoglycerate dehydrogenase gene causes severe neurodevelopmental defects and results in embryonic lethality. J. Biol. Chem. 279, 3573–3577 (2004)
S. Furuya, T. Tabata, J. Mitoma, K. Yamada, M. Yamasaki, A. Makino, T. Yamamoto, M. Watanabe, M. Kano, Y. Hirabayashi, L-serine and glycine serve as major astroglia-derived trophic factors for cerebellar purkinje neurons. Proc. Natl. Acad. Sci. U. S. A. 97, 11528–11533 (2000)
K. Yamasaki, K. Yamada, S. Furuya, J. Mitoma, Y. Hirabayashi, Y. Watanabe, 3-Phosphoglycerate dehydrogenase (3PGDH), a key enzyme for L-serine biosynthesis, is preferentially expressed in the radial glia/astrocyte lineage and olfactory ensheathing glia in the mouse brain. J. Neurosci. 21, 7691–7704 (2001)
Y. Achouri, M.H. Rider, E.V.V. Schaftingen, M. Robbi, Cloning, sequencing and expression of rat liver 3-phosphoglycerate dehydrogenase. Biochem. J. 323(2), 365–370 (1997)
K.L. Tobey, G.A. Grant, The nucleotide sequence of the serA gene of Escherichia coli and the amino acid sequence of the encoded protein, D-3-phosphoglycerate dehydrogenase. J. Biol. Chem. 261, 12179–12183 (1986)
R.D. Fleischmann, M.D. Adams, O. White, R.A. Clayton, E.F. Kirkness, A.R. Kerlavage, C.J. Bult, J.F. Tomb, B.A. Dougherty, J.M. Merrick, Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269, 496–512 (1995)
A. Sorokin, E. Zumstein, V. Azevedo, S.D. Ehrlich, P. Serror, The organization of the Bacillus subtilis 168 chromosome region between the spoVA and serA genetic loci, based on sequence data. Mol. Microbiol. 10, 385–395 (1993)
H.M. Cho, D.Y. Jun, M.A. Bae, Y.H. Kim, Nucleotide sequence and differential expression of the human 3-phosphoglycerate dehydrogenase gene. Gene 245, 193–201 (2000)
R. Wilson, R. Ainscough, K. Anderson, C. Baynes, M. Berks, J. Bonfield, J. Burton, M. Connell, T. Copsey, J. Cooper, 2.2 Mb of contiguous nucleotide sequence from chromosome III of C. elegans. Nature 368, 32–38 (1994)
J. Mitoma, S. Furuya, M. Shimizu, Y. Shinoda, K. Yoshida, N. Azuma, H. Tanaka, Y. Suzuki, Y. Hirabayashi, Mouse 3-phosphoglycerate dehydrogenase gene: genomic organization, chromosomal localization, and promoter analysis. Gene 334, 15–22 (2004)
M. Robbi, Y. Achouri, C. Szpirer, E. Van Schaftingen, The gene encoding rat 3-phosphoglycerate dehydrogenase. Mamm. Genome 11, 1034–1036 (2000)
S. Dey, Z. Hu, X.L. Xu, J.C. Sacchettini, G.A. Grant, D-3-Phosphoglycerate dehydrogenase from Mycobacterium tuberculosis is a link between the Escherichia coli and mammalian enzymes. J. Biol. Chem. 280, 14884–14891 (2005)
J.E. Unterlass, R.J. Wood, A. Baslé, J. Tucker, C. Cano, M.M.E. Noble, N.J. Curtin, Structural insights into the enzymatic activity and potential substrate promiscuity of human 3-phosphoglycerate dehydrogenase (PHGDH). Oncotarget 8, 104478–104491 (2017)
S. Dey, G.A. Grant, J.C. Sacchettini, Crystal structure of Mycobacterium tuberculosis D-3-phosphoglycerate dehydrogenase: extreme asymmetry in a tetramer of identical subunits. J. Biol. Chem. 280, 14892–14899 (2005)
D.J. Schuller, G.A. Grant, L.J. Banaszak, The allosteric ligand site in the Vmax-type cooperative enzyme phosphoglycerate dehydrogenase. Nat. Struct. Biol. 2, 69–76 (1995)
V. Truong, S. Huang, J. Dennis, M. Lemire, N. Zwingerman, D. Aïssi, I. Kassam, C. Perret, P. Wells, P.E. Morange, M. Wilson, D.A. Trégouët, F. Gagnon, Blood triglyceride levels are associated with DNA methylation at the serine metabolism gene PHGDH. Sci. Rep. 7, 11207 (2017)
R. Shaheen, Z. Rahbeeni, A. Alhashem, E. Faqeih, Q. Zhao, Y. Xiong, A. Almoisheer, S.M. Al-Qattan, H.A. Almadani, N. Al-Onazi, B.S. Al-Baqawi, M.A. Saleh, F.S. Alkuraya, Neu-Laxova syndrome, an inborn error of serine metabolism, is caused by mutations in PHGDH. Am. J. Hum. Genet. 94, 898–904 (2014)
D.J. Xiang, H.P. Yan, Q. Xin, F. Lu, X. Feng, Y. Zhao, Y. Liu, J.X. Yang, Cloning and expression of 3-phosphoglycerate dehydrogenase gene and its correlative antibodies in diagnosis of autoimmune hepatitis. Zhonghua Gan Zang Bing Za Zhi 17, 378–382 (2009)
J. Chen, F. Chung, G. Yang, M. Pu, H. Gao, W. Jiang, H. Yin, V. Capka, S. Kasibhatla, B. Laffitte, S. Jaeger, R. Pagliarini, Y. Chen, W. Zhou, Phosphoglycerate dehydrogenase is dispensable for breast tumor maintenance and growth. Oncotarget 4, 2502–2511 (2013)
R.B. Hamanaka, R. Nigdelioglu, A.Y. Meliton, Y. Tian, L.J. Witt, E. O’Leary, K.A. Sun, P.S. Woods, D. Wu, B. Ansbro, S. Ard, J.M. Rohde, N.O. Dulin, R.D. Guzy, G.M. Mutlu, Inhibition of phosphoglycerate dehydrogenase attenuates bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 58, 585–593 (2018)
H. Yoshino, N. Nohata, K. Miyamoto, M. Yonemori, T. Sakaguchi, S. Sugita, T. Itesako, S. Kofuji, M. Nakagawa, R. Dahiya, H. Enokida, PHGDH as a key enzyme for serine biosynthesis in HIF2α-targeting therapy for renal cell carcinoma. Cancer Res. 77, 6321–6329 (2017)
B. Zhang, A. Zheng, P. Hydbring, G. Ambroise, A.T. Ouchida, M. Goiny, H. Vakifahmetoglu-Norberg, E. Norberg, PHGDH defines a metabolic subtype in lung adenocarcinomas with poor prognosis. Cell Rep. 19, 2289–2303 (2017)
A.L. Engel, N.I. Lorenz, K. Klann, C. Münch, C. Depner, J.P. Steinbach, M.W. Ronellenfitsch, A.L. Luger, Serine-dependent redox homeostasis regulates glioblastoma cell survival. Br. J. Cancer 122(9), 1391–1398 (2020)
X.Q. Jia, S. Zhang, H.J. Zhu, W. Wang, J.H. Zhu, X.D. Wang, J.F. Qiang, Increased expression of PHGDH and prognostic significance in colorectal cancer. Transl. Oncol. 9, 191–196 (2016)
K. Snell, G. Weber, Enzymic imbalance in serine metabolism in rat hepatomas. Biochem. J. 233, 617–620 (1986)
K. Snell, Y. Natsumeda, J.N. Eble, J.L. Glover, G. Weber, Enzymic imbalance in serine metabolism in human colon carcinoma and rat sarcoma. Br. J. Cancer. 57, 87–90 (1988)
S. Noh, D.H. Kim, W.H. Jung, J.S. Koo, Expression levels of serine/glycine metabolism-related proteins in triple negative breast cancer tissues. Tumour Biol. 35, 4457–4468 (2014)
X. Zhang, W. Bai, Repression of phosphoglycerate dehydrogenase sensitizes triple-negative breast cancer to doxorubicin. Cancer Chemother. Pharmacol. 78, 655–659 (2016)
J. Fan, X. Teng, L. Liu, K.R. Mattaini, R.E. Looper, M.G. Vander Heiden, J.D. Rabinowitz, Human phosphoglycerate dehydrogenase produces the oncometabolite D-2-hydroxyglutarate. ACS Chem. Biol. 10, 510–516 (2015)
K.R. Mattaini, M.R. Sullivan, A.N. Lau, B.P. Fiske, R.T. Bronson, M.G. Vander Heiden, Increased PHGDH expression promotes aberrant melanin accumulation. BMC Cancer 19, 723 (2019)
Y. Ou, S.J. Wang, L. Jiang, B. Zheng, W. Gu, p53 protein-mediated regulation of phosphoglycerate dehydrogenase (PHGDH) is crucial for the apoptotic response upon serine starvation. J. Biol. Chem. 290, 457–466 (2015)
J. Liu, S. Guo, Q. Li, L. Yang, Z. Xia, L. Zhang, Z. Huang, N. Zhang, Phosphoglycerate dehydrogenase induces glioma cells proliferation and invasion by stabilizing forkhead box M1. J. Neurooncol. 111, 245–255 (2013)
Z. Jing, W. Heng, D. Aiping, Q. Yafei, Z. Shulan, Expression and clinical significance of phosphoglycerate dehydrogenase and squamous cell carcinoma antigen in cervical cancer. Int. J. Gynecol. Cancer 23, 1465–1469 (2013)
Z. Jing, W. Heng, L. Xia, W. Ning, Q. Yafei, Z. Yao, Z. Shulan, Downregulation of phosphoglycerate dehydrogenase inhibits proliferation and enhances cisplatin sensitivity in cervical adenocarcinoma cells by regulating Bcl-2 and caspase-3. Cancer Biol. Ther. 16, 541–548 (2015)
J. Zhu, J. Ma, X. Wang, T. Ma, S. Zhang, W. Wang, X. Zhou, J. Shi, High Expression of PHGDH predicts poor prognosis in non-small cell lung cancer. Transl. Oncol. 9, 592–599 (2016)
X. Ma, B. Li, J. Liu, Y. Fu, Y. Luo, Phosphoglycerate dehydrogenase promotes pancreatic cancer development by interacting with eIF4A1 and eIF4E. J. Exp. Clin. Cancer Res. 38, 66 (2019)
Z. Song, C. Feng, Y. Lu, Y. Lin, C. Dong, PHGDH is an independent prognosis marker and contributes cell proliferation, migration and invasion in human pancreatic cancer. Gene 642, 43–50 (2018)
Y. Xian, S. Zhang, X. Wang, J. Qin, W. Wang, H. Wu, Phosphoglycerate dehydrogenase is a novel predictor for poor prognosis in gastric cancer. Onco. Targets Ther. 9, 5553–5560 (2016)
A. Fenner, Kidney cancer: PHGDH is key for targeting HIF in RCC. Nat. Rev. Urol. 14, 702 (2017)
Y. Ye, Y. Zhou, L. Zhang, Y. Chen, X. Lyu, L. Cai, Y. Lu, Y. Deng, J. Wang, K. Yao, W. Fang, H. Cai, X. Li, EBV-miR-BART1 is involved in regulating metabolism-associated genes in nasopharyngeal carcinoma. Biochem. Biophys. Res. Commun. 436, 19–24 (2013)
W.Y. Sun, H.M. Kim, W.H. Jung, J.S. Koo, Expression of serine/glycine metabolism-related proteins is different according to the thyroid cancer subtype. J. Transl. Med. 14, 168 (2016)
J. Wang, H. Ni, L. Chen, Y.X. Liu, C.B. Chen, W.Q. Song, Preparation and analysis of cSNP chip on hepatocellular carcinoma-related genes. HBPD Int. 4, 398–402 (2005)
L. Wei, D. Lee, C.T. Law, M.S. Zhang, J. Shen, D.W. Chin, A. Zhang, F.H. Tsang, C.L. Wong, I.O. Ng, C.C. Wong, C.M. Wong, Genome-wide CRISPR/Cas9 library screening identified PHGDH as a critical driver for Sorafenib resistance in HCC. Nat. Commun. 10, 4681 (2019)
J.K. Dong, H.M. Lei, Q. Liang, Y.B. Tang, Y. Zhou, Y. Wang, S. Zhang, W.B. Li, Y. Tong, G. Zhuang, L. Zhang, H.Z. Chen, L. Zhu, Y. Shen, Overcoming erlotinib resistance in EGFR mutation-positive lung adenocarcinomas through repression of phosphoglycerate dehydrogenase. Theranostics 8, 1808–1823 (2018)
S. Yoon, J.G. Kim, A.N. Seo, S.Y. Park, H.J. Kim, J.S. Park, G.S. Choi, J.Y. Jeong, Y. Jun do, G.S. Yoon, B.W. Kang, Clinical implication of serine metabolism-associated enzymes in colon cancer. Oncology 89, 351–359 (2015)
F. Polet, C. Corbet, A. Pinto, L.I. Rubio, R. Martherus, V. Bol, X. Drozak, V. Grégoire, O. Riant, O. Feron, Reducing the serine availability complements the inhibition of the glutamine metabolism to block leukemia cell growth. Oncotarget 7, 1765–1776 (2016)
X. Wu, J. Xia, J. Zhang, Y. Zhu, Y. Wu, J. Guo, S. Chen, Q. Lei, B. Meng, C. Kuang, X. Feng, Y. He, Y. Shen, X. Li, L. Qiu, G. Li, W. Zhou, Phosphoglycerate dehydrogenase promotes proliferation and bortezomib resistance through increasing reduced glutathione synthesis in multiple myeloma. Br. J. Haematol. 190, 52–66 (2020)
A. Palermo, M. Fosca, G. Tabacco, F. Marini, V. Graziani, M.C. Santarsia, F. Longo, A. Lauria, R. Cesareo, I. Giovannoni, C. Taffon, M. Rocchia, S. Manfrini, P. Crucitti, P. Pozzilli, A. Crescenzi, J.V. Rau, Raman spectroscopy applied to parathyroid tissues: a new diagnostic tool to discriminate normal tissue from adenoma. Anal. Chem. 90(1), 847–854 (2018)
A. di Masi, L. Leboffe, A. Sodo, G. Tabacco, R. Cesareo, M. Sbroscia, I. Giovannoni, C. Taffon, P. Crucitti, F. Longo, S. Manfrini, M.A. Ricci, P. Ascenzi, A. Crescenzi, A. Palermo, Metabolic profile of human parathyroid adenoma. Endocrine 67(3), 699–707 (2020)
I. Gromova, P. Gromov, N. Honma, S. Kumar, D. Rimm, M.L. Talman, V.T. Wielenga, J.M. Moreira, High level PHGDH expression in breast is predominantly associated with keratin 5-positive cell lineage independently of malignancy. Mol. Oncol. 9, 1636–1654 (2015)
E. Mullarky, N.C. Lucki, R. Beheshti Zavareh, J.L. Anglin, A.P. Gomes, B.N. Nicolay, J.C. Wong, S. Christen, H. Takahashi, P.K. Singh, J. Blenis, J.D. Warren, S.M. Fendt, J.M. Asara, G.M. DeNicola, C.A. Lyssiotis, L.L. Lairson, L.C. Cantley, Identification of a small molecule inhibitor of 3-phosphoglycerate dehydrogenase to target serine biosynthesis in cancers. Proc. Natl. Acad. Sci. U. S. A. 113, 1778–1783 (2016)
M.E. Pacold, K.R. Brimacombe, S.H. Chan, J.M. Rohde, C.A. Lewis, L.J. Swier, R. Possemato, W.W. Chen, L.B. Sullivan, B.P. Fiske, S. Cho, E. Freinkman, K. Birsoy, M. Abu-Remaileh, Y.D. Shaul, C.M. Liu, M. Zhou, M.J. Koh, H. Chung, S.M. Davidson, A. Luengo, A.Q. Wang, X. Xu, A. Yasgar, L. Liu, G. Rai, K.D. Westover, M.G. Vander Heiden, M. Shen, N.S. Gray, M.B. Boxer, D.M. Sabatini, A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nat. Chem. Biol. 12, 452–458 (2016)
J.M. Rohde, K.R. Brimacombe, L. Liu, M.E. Pacold, A. Yasgar, D.M. Cheff, T.D. Lee, G. Rai, B. Baljinnyam, Z. Li, A. Simeonov, M.D. Hall, M. Shen, D.M. Sabatini, M.B. Boxer, Discovery and optimization of piperazine-1-thiourea-based human phosphoglycerate dehydrogenase inhibitors. Bioorg. Med. Chem. 26, 1727–1739 (2018)
J.E. Unterlass, A. Baslé, T.J. Blackburn, J. Tucker, C. Cano, M.E.M. Noble, N.J. Curtin, Validating and enabling phosphoglycerate dehydrogenase (PHGDH) as a target for fragment-based drug discovery in PHGDH-amplified breast cancer. Oncotarget 9, 13139–13153 (2018)
The 5th RSC-BMCS fragment-based drug discovery meeting at Churchill College, Cambridge (UK) (2015)
N. Fuller, L. Spadola, S. Cowen, J. Patel, H. Schönherr, Q. Cao, A. McKenzie, F. Edfeldt, A. Rabow, R. Goodnow, An improved model for fragment-based lead generation at AstraZeneca. Drug Discov. Today 21, 1272–1283 (2016)
S. Ravez, C. Corbet, Q. Spillier, A. Dutu, A.D. Robin, E. Mullarky, L.C. Cantley, O. Feron, R. Frédérick, α-Ketothioamide derivatives: a promising tool to interrogate phosphoglycerate dehydrogenase (PHGDH). J. Med. Chem. 60, 1591–1597 (2017)
Q. Wang, M.V. Liberti, P. Liu, X. Deng, Y. Liu, J.W. Locasale, L. Lai, Rational design of selective allosteric inhibitors of PHGDH and serine synthesis with anti-tumor activity. Cell Chem. Biol. 24, 55–65 (2017)
J. Guo, X. Gu, M. Zheng, Y. Zhang, L. Chen, H. Li, Azacoccone E inhibits cancer cell growth by targeting 3-phosphoglycerate dehydrogenase. Bioorg. Chem. 87, 16–22 (2019)
M. Zheng, J. Guo, J. Xu, K. Yang, R. Tang, X. Gu, H. Li, L. Chen, Ixocarpalactone A from dietary tomatillo inhibits pancreatic cancer growth by targeting PHGDH. Food Funct. 10(6), 3386–3395 (2019)
D. González-Mendoza, D. Ascencio-Martinez, A.H. Poox, V. Mendez-Trujillo, O. Grimaldo-Juarez, J.F.S. Hernández, L.C. Diaz, S.M.A. Marin, Phenolic compounds and physiochemical analysis of Physalis ixocarpa genotypes. Sci. Res. Essays 6, 3808–3814 (2011)
E. Mullarky, J. Xu, A.D. Robin, D.J. Huggins, A. Jennings, N. Noguchi, A. Olland, D. Lakshminarasimhan, M. Miller, D. Tomita, M. Michino, T. Su, G. Zhang, A.W. Stamford, P.T. Meinke, S. Kargman, L.C. Cantley, Inhibition of 3-phosphoglycerate dehydrogenase (PHGDH) by indole amides abrogates de novo serine synthesis in cancer cells. Bioorg. Med. Chem. Lett. 29, 2503–2510 (2019)
H. Weinstabl, M. Treu, J. Rinnenthal, S.K. Zahn, P. Ettmayer, G. Bader, G. Dahmann, D. Kessler, K. Rumpel, N. Mischerikow, F. Savarese, T. Gerstberger, M. Mayer, A. Zoephel, R. Schnitzer, W. Sommergruber, P. Martinelli, H. Arnhof, B. Peric-Simov, K.S. Hofbauer, G. Garavel, Y. Scherbantin, S. Mitzner, T.N. Fett, G. Scholz, J. Bruchhaus, M. Burkard, R. Kousek, T. Ciftci, B. Sharps, A. Schrenk, C. Harrer, D. Haering, B. Wolkerstorfer, X. Zhang, X. Lv, A. Du, D. Li, Y. Li, J. Quant, M. Pearson, D.B. McConnell, Intracellular trapping of the selective phosphoglycerate dehydrogenase (PHGDH) inhibitor disrupts serine biosynthesis. J. Med. Chem. 62, 7976–7997 (2019)
E. Saiah, 3-phosphoglycerate dehydrogenase inhibitors and uses thereof. Patents. (2016)
Q. Spillier, D. Vertommen, S. Ravez, R. Marteau, Q. Thémans, C. Corbet, O. Feron, J. Wouters, R. Frédérick, Anti-alcohol abuse drug disulfiram inhibits human PHGDH via disruption of its active tetrameric form through a specific cysteine oxidation. Sci. Rep. 9, 4737 (2019)
J. Son, C.A. Lyssiotis, H. Ying, X. Wang, S. Hua, M. Ligorio, R.M. Perera, C.R. Ferrone, E. Mullarky, N. Shyh-Chang, Y. Kang, J.B. Fleming, N. Bardeesy, J.M. Asara, M.C. Haigis, R.A. DePinho, L.C. Cantley, A.C. Kimmelman, Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101–105 (2013)
E.A. Zaal, W. Wu, G. Jansen, S. Zweegman, J. Cloos, C.R. Berkers, Bortezomib resistance in multiple myeloma is associated with increased serine synthesis. Cancer Metab. 5, 7 (2017)
C.K. Zogg, Phosphoglycerate dehydrogenase: potential therapeutic target and putative metabolic oncogene. J. Oncol. 2014, 524101 (2014)
M.A. Reid, A.E. Allen, S. Liu, M.V. Liberti, P. Liu, X. Liu, Z. Dai, X. Gao, Q. Wang, Y. Liu, L. Lai, J.W. Locasale, Serine synthesis through PHGDH coordinates nucleotide levels by maintaining central carbon metabolism. Nat. Commun. 9, 5442 (2018)
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC) [NOs. 81773594, U1803122, U1703111, 81473254, and 81773637], the National Mega-project for Innovative Drugs (grant number 2019ZX09721001-004-007), the National Science and Technology Major Project of the Ministry of Science and Technology of China (No. 2018ZX09735005), the Chunhui Program-Cooperative Research Project of the Ministry of Education, Liaoning Province Natural Science Foundation (NO. 2020-MZLH-31, 2019-MS-299) and the Liaoning Revitalization Talents Program (NO. XLYC1807182). We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Yes.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mingxue Li and Canrong Wu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, M., Wu, C., Yang, Y. et al. 3-Phosphoglycerate dehydrogenase: a potential target for cancer treatment. Cell Oncol. 44, 541–556 (2021). https://doi.org/10.1007/s13402-021-00599-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-021-00599-9