Abstract
Purpose
Approximately 20% of all salivary gland cancer patients who are treated with current treatment modalities will ultimately develop metastases. Its most common form, mucoepidermoid carcinoma (MEC) is a highly aggressive tumor with an overall 5-year survival rate of ~30%. Until now, several chemotherapeutic drugs have been tested for the treatment of salivary gland tumors, but the results have been disappointing and the drugs often cause unwanted side effects. Therefore, several recent studies have focused on the potential of alternative and/or complementary therapeutic options, including the use of silymarin.
Methods
The effects of silymarin and its active component silibinin on salivary gland cancer-derived MC3 and HN22 cells and their underlying molecular mechanisms were examined using trypan blue exclusion, 4′-6-diamidino-2-phenylindole (DAPI) staining, Live/Dead, Annexin V/PI staining, mitochondrial membrane potential (ΔΨm) measurement, quantitative RT-PCR, soft agar colony formation and Western blotting analyses.
Results
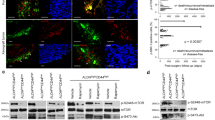
We found that silymarin and silibinin dramatically increased the expression of the pro-apoptotic protein Bim in a concentration- and time-dependent manner and, concomitantly, induced apoptosis in MC3 and HN22 cells. We also found that ERK1/2 signaling inhibition successfully sensitized these cells to the apoptotic effects of silymarin and silibinin, which indicates that the ERK1/2 signaling pathway may act as an upstream regulator that modulates the silymarin/silibinin-induced Bim signaling pathway.
Conclusions
Taken together, we conclude that ERK1/2 signaling pathway inhibition by silymarin and silibinin increases the expression of the pro-apoptotic Bcl-2 family member Bim which, subsequently, induces mitochondria-mediated apoptosis in salivary gland cancer-derived cells.







Similar content being viewed by others
References
B. Lujan, S. Hakim, S. Moyano, A. Nadal, M. Caballero, A. Diaz, A. Valera, M. Carrera, A. Cardesa, L. Alos, Activation of the EGFR/ERK pathway in high-grade mucoepidermoid carcinomas of the salivary glands. Br. J. Cancer 103, 510–516 (2010)
S. Schwarz, C. Stiegler, M. Muller, T. Ettl, G. Brockhoff, J. Zenk, A. Agaimy, Salivary gland mucoepidermoid carcinoma is a clinically, morphologically and genetically heterogeneous entity: a clinicopathological study of 40 cases with emphasis on grading, histological variants and presence of the t(11;19) translocation. Histopathology 58, 557–570 (2011)
M.J. Hicks, A.K. el-Naggar, C.M. Flaitz, M.A. Luna, J.G. Batsakis, Histocytologic grading of mucoepidermoid carcinoma of major salivary glands in prognosis and survival: a clinicopathologic and flow cytometric investigation. Head Neck 17, 89–95 (1995)
M. Guzzo, S. Andreola, G. Sirizzotti, G. Cantu, Mucoepidermoid carcinoma of the salivary glands: clinicopathologic review of 108 patients treated at the National Cancer Institute of Milan. Ann. Surg. Oncol. 9, 688–695 (2002)
E. Vattemi, C. Graiff, T. Sava, R. Pedersini, A. Caldara, M. Mandara, Systemic therapies for recurrent and/or metastatic salivary gland cancers. Expert. Rev. Anticancer. Ther. 8, 393–402 (2008)
L. Agoni, I. Basu, S. Gupta, A. Alfieri, A. Gambino, G.L. Goldberg, E.P. Reddy, C. Guha, Rigosertib is a more effective radiosensitizer than cisplatin in concurrent chemoradiation treatment of cervical carcinoma, in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 88, 1180–1187 (2014)
C.D. Scripture, W.D. Figg, A. Sparreboom, Peripheral neuropathy induced by paclitaxel: Recent insights and future perspectives. Curr. Neuropharmacol. 4, 165–172 (2006)
S.C. Pradhan, C. Girish, Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J. Med. Res. 124, 491–504 (2006)
N. Vargas-Mendoza, E. Madrigal-Santillan, A. Morales-Gonzalez, J. Esquivel-Soto, C. Esquivel-Chirino, Y.G.-R.M. Garcia-Luna, J.A. Gayosso-de-Lucio, J.A. Morales-Gonzalez, Hepatoprotective effect of silymarin. World J. Hepatol. 6, 144–149 (2014)
T.W. Flaig, D.L. Gustafson, L.J. Su, J.A. Zirrolli, F. Crighton, G.S. Harrison, A.S. Pierson, R. Agarwal, L.M. Glode, A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Investig. New Drugs 25, 139–146 (2007)
F.H. Schroder, M.J. Roobol, E.R. Boeve, R. de Mutsert, S.D. Zuijdgeest-van Leeuwen, I. Kersten, M.F. Wildhagen and A. van Helvoort, Randomized, double-blind, placebo-controlled crossover study in men with prostate cancer and rising PSA: effectiveness of a dietary supplement. Eur. Urol. 48, 922–930; discussion 930–921 (2005)
G. Scambia, R. De Vincenzo, F.O. Ranelletti, P.B. Panici, G. Ferrandina, G. D'Agostino, A. Fattorossi, E. Bombardelli, S. Mancuso, Antiproliferative effect of silybin on gynaecological malignancies: synergism with cisplatin and doxorubicin. Eur. J. Cancer 32A, 877–882 (1996)
X. Zi, D.K. Feyes, R. Agarwal, Anticarcinogenic effect of a flavonoid antioxidant, silymarin, in human breast cancer cells MDA-MB 468: induction of G1 arrest through an increase in Cip1/p21 concomitant with a decrease in kinase activity of cyclin-dependent kinases and associated cyclins. Clin. Cancer Res. 4, 1055–1064 (1998)
G. Sharma, R.P. Singh, D.C. Chan, R. Agarwal, Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells. Anticancer Res. 23, 2649–2655 (2003)
G. Deep, R. Agarwal, Chemopreventive efficacy of silymarin in skin and prostate cancer. Integr. Cancer Ther. 6, 130–145 (2007)
A. Tyagi, K. Raina, R.P. Singh, M. Gu, C. Agarwal, G. Harrison, L.M. Glode, R. Agarwal, Chemopreventive effects of silymarin and silibinin on N-butyl-N-(4-hydroxybutyl) nitrosamine induced urinary bladder carcinogenesis in male ICR mice. Mol. Cancer Ther. 6, 3248–3255 (2007)
A. Tyagi, C. Agarwal, G. Harrison, L.M. Glode, R. Agarwal, Silibinin causes cell cycle arrest and apoptosis in human bladder transitional cell carcinoma cells by regulating CDKI-CDK-cyclin cascade, and caspase 3 and PARP cleavages. Carcinogenesis 25, 1711–1720 (2004)
R. Invernizzi, S. Bernuzzi, D. Ciani, E. Ascari, Silymarine during maintenance therapy of acute promyelocytic leukemia. Haematologica 78, 340–341 (1993)
K. Ramasamy, R. Agarwal, Multitargeted therapy of cancer by silymarin. Cancer Lett. 269, 352–362 (2008)
R. Agarwal, C. Agarwal, H. Ichikawa, R.P. Singh, B.B. Aggarwal, Anticancer potential of silymarin: from bench to bed side. Anticancer Res. 26, 4457–4498 (2006)
K. Balmanno, S.J. Cook, Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 16, 368–377 (2009)
J.F. Ohren, H. Chen, A. Pavlovsky, C. Whitehead, E. Zhang, P. Kuffa, C. Yan, P. McConnell, C. Spessard, C. Banotai, W.T. Mueller, A. Delaney, C. Omer, J. Sebolt-Leopold, D.T. Dudley, I.K. Leung, C. Flamme, J. Warmus, M. Kaufman, S. Barrett, H. Tecle, C.A. Hasemann, Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat. Struct. Mol. Biol. 11, 1192–1197 (2004)
J.A. Wickenden, H. Jin, M. Johnson, A.S. Gillings, C. Newson, M. Austin, S.D. Chell, K. Balmanno, C.A. Pritchard, S.J. Cook, Colorectal cancer cells with the BRAF(V600E) mutation are addicted to the ERK1/2 pathway for growth factor-independent survival and repression of BIM. Oncogene 27, 7150–7161 (2008)
M.S. Cragg, E.S. Jansen, M. Cook, C. Harris, A. Strasser, C.L. Scott, Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic. J. Clin. Invest. 118, 3651–3659 (2008)
E.S. Choi, J.Y. Jung, J.S. Lee, J.H. Park, N.P. Cho, S.D. Cho, Myeloid cell leukemia-1 is a key molecular target for mithramycin A-induced apoptosis in androgen-independent prostate cancer cells and a tumor xenograft animal model. Cancer Lett. 328, 65–72 (2013)
P. Li, D. Nijhawan, I. Budihardjo, S.M. Srinivasula, M. Ahmad, E.S. Alnemri, X. Wang, Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489 (1997)
P.X. Petit, N. Zamzami, J.L. Vayssiere, B. Mignotte, G. Kroemer, M. Castedo, Implication of mitochondria in apoptosis. Mol. Cell. Biochem. 174, 185–188 (1997)
S. Shimizu, M. Narita, Y. Tsujimoto, Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399, 483–487 (1999)
H. Puthalakath, D.C. Huang, L.A. O'Reilly, S.M. King, A. Strasser, The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell 3, 287–296 (1999)
M. Rosner, M. Hengstschlager, Cytoplasmic and nuclear distribution of the protein complexes mTORC1 and mTORC2: rapamycin triggers dephosphorylation and delocalization of the mTORC2 components rictor and sin1. Hum. Mol. Genet. 17, 2934–2948 (2008)
R. Hughes, J. Gilley, M. Kristiansen, J. Ham, The MEK-ERK pathway negatively regulates bim expression through the 3′ UTR in sympathetic neurons. BMC Neurosci. 12, 69 (2011)
F. Luciano, A. Jacquel, P. Colosetti, M. Herrant, S. Cagnol, G. Pages, P. Auberger, Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene 22, 6785–6793 (2003)
N. Cidlinsky, G. Dogliotti, T. Pukrop, R. Jung, F. Weber, M.P. Krahn, Inactivation of the LKB1-AMPK signaling pathway does not contribute to salivary gland tumor development - a short report. Cell. Oncol. 39, 389–396 (2016)
K. Ho, H. Lin, D.K. Ann, P.G. Chu, Y. Yen, An overview of the rare parotid gland cancer. Head Neck Oncol. 3, 40 (2011)
I. Schwentner, P. Obrist, W. Thumfart, G. Sprinzl, Distant metastasis of parotid gland tumors. Acta Otolaryngol. 126, 340–345 (2006)
S.A. Laurie, L. Licitra, Systemic therapy in the palliative management of advanced salivary gland cancers. J. Clin. Oncol. 24, 2673–2678 (2006)
J.K. Prasain, S. Barnes, Metabolism and bioavailability of flavonoids in chemoprevention: current analytical strategies and future prospectus. Mol. Pharm. 4, 846–864 (2007)
H. Nishino, Y. Satomi, H. Tokuda, M. Masuda, Cancer control by phytochemicals. Curr. Pharm. Des. 13, 3394–3399 (2007)
J. Post-White, E.J. Ladas, K.M. Kelly, Advances in the use of milk thistle (Silybum marianum). Integr. Cancer Ther. 6, 104–109 (2007)
C. Hoh, D. Boocock, T. Marczylo, R. Singh, D.P. Berry, A.R. Dennison, D. Hemingway, A. Miller, K. West, S. Euden, G. Garcea, P.B. Farmer, W.P. Steward, A.J. Gescher, Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences. Clin. Cancer Res. 12, 2944–2950 (2006)
A. Gross, J.M. McDonnell, S.J. Korsmeyer, BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13, 1899–1911 (1999)
A.K. Berger, G.P. Cortese, K.D. Amodeo, A. Weihofen, A. Letai, M.J. LaVoie, Parkin selectively alters the intrinsic threshold for mitochondrial cytochrome c release. Hum. Mol. Genet. 18, 4317–4328 (2009)
A. Tyagi, R.P. Singh, C. Agarwal, R. Agarwal, Silibinin activates p53-caspase 2 pathway and causes caspase-mediated cleavage of Cip1/p21 in apoptosis induction in bladder transitional-cell papilloma RT4 cells: evidence for a regulatory loop between p53 and caspase 2. Carcinogenesis 27, 2269–2280 (2006)
S.K. Katiyar, A.M. Roy, M.S. Baliga, Silymarin induces apoptosis primarily through a p53-dependent pathway involving Bcl-2/Bax, cytochrome c release, and caspase activation. Mol. Cancer Ther. 4, 207–216 (2005)
K. Ohshiro, S.K. Rayala, M.D. Williams, R. Kumar, A.K. El-Naggar, Biological role of estrogen receptor beta in salivary gland adenocarcinoma cells. Clin. Cancer Res. 12, 5994–5999 (2006)
P. Meier, G. Evan, Dying like flies. Cell 95, 295–298 (1998)
Z. Xia, M. Dickens, J. Raingeaud, R.J. Davis, M.E. Greenberg, Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270, 1326–1331 (1995)
R. Ley, K. Balmanno, K. Hadfield, C. Weston, S.J. Cook, Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein Bim. J. Biol. Chem. 278, 18811–18816 (2003)
R. Ley, K.E. Ewings, K. Hadfield, E. Howes, K. Balmanno, S.J. Cook, Extracellular signal-regulated kinases 1/2 are serum-stimulated "Bim(EL) kinases" that bind to the BH3-only protein Bim(EL) causing its phosphorylation and turnover. J. Biol. Chem. 279, 8837–8847 (2004)
G. Mallikarjuna, S. Dhanalakshmi, R.P. Singh, C. Agarwal, R. Agarwal, Silibinin protects against photocarcinogenesis via modulation of cell cycle regulators, mitogen-activated protein kinases, and Akt signaling. Cancer Res. 64, 6349–6356 (2004)
N. Bhatia, J. Zhao, D.M. Wolf, R. Agarwal, Inhibition of human carcinoma cell growth and DNA synthesis by silibinin, an active constituent of milk thistle: comparison with silymarin. Cancer Lett. 147, 77–84 (1999)
R.P. Singh, S. Dhanalakshmi, S. Mohan, C. Agarwal, R. Agarwal, Silibinin inhibits UVB- and epidermal growth factor-induced mitogenic and cell survival signaling involving activator protein-1 and nuclear factor-kappaB in mouse epidermal JB6 cells. Mol. Cancer Ther. 5, 1145–1153 (2006)
J.C. Lee, L.C. Chung, Y.J. Chen, T.H. Feng, W.T. Chen, H.H. Juang, Upregulation of B-cell translocation gene 2 by epigallocatechin-3-gallate via p38 and ERK signaling blocks cell proliferation in human oral squamous cell carcinoma cells. Cancer Lett. 360, 310–318 (2015)
C.R. Weston, K. Balmanno, C. Chalmers, K. Hadfield, S.A. Molton, R. Ley, E.F. Wagner, S.J. Cook, Activation of ERK1/2 by deltaRaf-1:ER* represses Bim expression independently of the JNK or PI3K pathways. Oncogene 22, 1281–1293 (2003)
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A1A2055874 and 2016R1A2B4006794).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Choi, ES., Oh, S., Jang, B. et al. Silymarin and its active component silibinin act as novel therapeutic alternatives for salivary gland cancer by targeting the ERK1/2-Bim signaling cascade. Cell Oncol. 40, 235–246 (2017). https://doi.org/10.1007/s13402-017-0318-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-017-0318-8