Abstract
Purpose
Hesperidin, a glycoside flavonoid, is thought to act as an anti-cancer agent, since it has been found to exhibit both pro-apoptotic and anti-proliferative effects in several cancer cell types. The mechanisms underlying hesperidin-induced growth arrest and apoptosis are, however, not well understood. Here, we aimed to investigate the anti-proliferative and apoptotic effects of hesperidin on non-small cell lung cancer (NSCLC) cells and to investigate the mechanisms involved.
Methods
The anti-proliferative and apoptotic effects of hesperidin on two NSCLC-derived cell lines, A549 and NCI-H358, were determined using a WST-1 colorimetric assay, a LDH cytotoxicity assay, a Cell Death Detection assay, an AnnexinV-FITC assay, a caspase-3 assay and a JC-1 assay, respectively, all in a time- and dose-dependent manner. As a control, non-cancerous MRC-5 lung fibroblasts were included. Changes in whole genome gene expression profiles were assessed using an Illumina Human HT-12v4 beadchip microarray platform, and subsequent data analyses were performed using an Illumina Genome Studio and Ingenuity Pathway Analyser (IPA).
Results
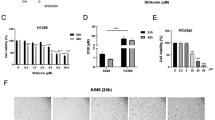
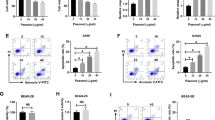
We found that after hesperidin treatment, A549 and NCI-H358 cells exhibited decreasing cell proliferation and increasing caspase-3 and other apoptosis-related activities, in conjunction with decreasing mitochondrial membrane potential activities, in a dose- and time-dependent manner. Through a GO analysis, by which changes in gene expression profiles were compared, we found that the FGF and NF-κB signal transduction pathways were most significantly affected in the hesperidin treated NCI-H358 and A549 NSCLC cells.
Conclusions
Our results indicate that hesperidin elicits an in vitro growth inhibitory effect on NSCLC cells by modulating immune response-related pathways that affect apoptosis. When confirmed in vivo, hesperidin may serve as a novel anti-proliferative agent for non-small cell lung cancer.






Similar content being viewed by others
Abbreviations
- EF:
-
Nucleosomal enrichment factor
- FBS:
-
Fetal bovine serum
- FGF:
-
Fibroblast growth factor
- FITC:
-
Fluorescein ısothiocyanate
- IPA:
-
Ingenuity pathway analysis
- LDH:
-
Lactate dehydrogenase
- MEM-α:
-
Eagle’s minimum essential medium
- NF-kB:
-
Nuclear factor kappa B
- NSCLC:
-
Non-small cell lung cancer
- PS:
-
Phosphatidylserine
- RPMI-1640:
-
Roswell Park Memorial Institute-1640
References
W. Pao, N. Girard, New driver mutations in non-small-cell lung cancer. Lancet Oncol. 12, 175–180 (2011)
W.D. Travis, Classification of lung cancer. Semin. Roentgenol. 46, 178–186 (2011)
Q. Wu, Y.F. Chen, J. Fu, Q.H. You, S.M. Wang, X. Huang, X.J. Feng, S.H. Zhang, Short hairpin RNA-mediated down-regulation of CENP-A attenuates the aggressive phenotype of lung adenocarcinoma cells. Cell. Oncol. 37, 399–407 (2014)
A. Koren, H. Motaln, T. Cufer, Lung cancer stem cells: a biological and clinical perspective. Cell. Oncol. 36, 265–275 (2013)
N. Peled, M.W. Wynes, N. Ikeda, T. Ohira, K. Yoshida, J. Qian, M. Ilouze, R. Brenner, Y. Kato, C. Mascaux, F.R. Hirsch, Insulin-like growth factor-1 receptor (IGF-1R) as a biomarker for resistance to the tyrosine kinase inhibitor gefitinib in non-small cell lung cancer. Cell. Oncol. 36, 277–288 (2013)
A. Maier, AL. Peille, V. Vuaroqueaux, M. Lahn. Anti-tumor activity of the TGF-β receptor kinase inhibitor galunisertib (LY2157299 monohydrate) in patient-derived tumor xenografts. Cell. Oncol. 2015 Jan 9. [Epub ahead of print] DOI 10.1007/s13402-014-0210-8
P. Ulivi, R. Silvestrini, Role of quantitative and qualitative characteristics of free circulating DNA in the management of patients with non-small cell lung cancer. Cell. Oncol. 36, 439–448 (2013)
G. Giaccone, Twenty-five years of treating advanced NSCLC: what have we achieved? Ann. Oncol. 15 Suppl 4: iv81–83 (2004)
J.W. Neal, L.V. Sequist, Exciting new targets in lung cancer therapy: ALK, IGF-1R, HDAC, and Hh. Curr. Treat. Options in Oncol. 11, 36–44 (2010)
R. Sangha, P.N. Lara, P.C. Mack, D.R. Gandara, Beyond antiepidermal growth factor receptors and antiangiogenesis strategies for nonsmall cell lung cancer: exploring a new frontier. Curr. Opin. Oncol. 21, 116–123 (2009)
S. Mateen, K. Raina, R. Agarwal, Chemopreventive and anti-cancer efficacy of silibinin against growth and progression of lung cancer. Nutr. Cancer J. 65, 3–11 (2013)
J. Nones, T.C.E. Spohr, F.C. Gomes, Hesperidin, a flavone glycoside, as mediator of neuronal survival. Neurochem. Res. 36, 1776–1784 (2011)
T. Tanaka, R. Takahashi, Flavonoids and asthma. Nutrients 10, 2128–2143 (2013)
J.A. Manthey, K. Grohmann, N. Guthrie, Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr. Med. Chem. 8, 135–153 (2001)
J. Yu, L. Wang, R.L. Walzem, E.G. Miller, L.M. Pike, B.S. Patil, Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J. Agric. Food Chem. 53, 2009–2014 (2005)
S.L. Hwang, P.H. Shih, G.C. Yen, Neuroprotective effects of citrus flavonoids. J. Agric. Food Chem. 60, 877–885 (2012)
E. Meiyanto, A. Hermawan, Anindyajati: natural products for cancer-targeted therapy: citrus flavonoids as potent chemopreventive agents. Asian. Pac. J. Cancer Prev. 13, 427–1436 (2012)
J.A. Manthey, N. Guthrie, Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 50, 5837–5843 (2002)
G. Saiprasad, P. Chitra, R. Manikandan, G. Sudhandiran, Hesperidin alleviates oxidative stress and downregulates the expressions of proliferative and inflammatory markers in azoxymethane-induced experimental colon carcinogenesis in mice. Inflamm. Res. 62, 425–440 (2013)
O.G. Benavente, J. Castillo, M. Alcaraz, V. Vicente, J.A. Del, A. Ortuno, Beneficial action of Citrus flavonoids on multiple cancer-related biological pathways. Curr. Cancer Drug Targets 7, 795–809 (2007)
J. Nones, T.C.E. Spohr, F.C. Gomes, Hesperidin, a flavone glycoside, as mediator of neuronal survival. Neurochem. Res. 36, 1776–1784 (2011)
V. Gaur, A. Kumar, Hesperidin pre-treatment attenuates NO-mediated cerebral ischemic reperfusion injury and memory dysfunction. Pharmacol. Rep. 62, 635–648 (2010)
S. Ou, Pharmacological action of hesperidin. Zhong. Yao. Cai. 25, 531–533 (2002)
O. Benavente-Garcia, J. Castillo, Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 56, 6185–6205 (2008)
A. Chanet, D. Milenkovic, C. Manach, A. Mazur, C. Morand, Citrus flavanones: what is their role in cardiovascular protection? J. Agric. Food Chem. 60, 8809–8822 (2012)
B.D. Sahu, M. Kuncha, G.J. Sindhura, R. Sistla, Hesperidin attenuates cisplatin-induced acute renal injury by decreasing oxidative stress, inflammation and DNA damage. Phytomedicine 20, 453–460 (2013)
J.R. Patil, K.N. Chidambara Murthy, G.K. Jayaprakasha, M.B. Chetti, B.S. Patil, Bioactive compounds from Mexican lime [Citrus aurantifolia] juice induce apoptosis in human pancreatic cells. J. Agric. Food Chem 57, 10933–10942 (2009)
M. Galleano, O. Pechanova, C.G. Fraga, Hypertension, nitric oxide, oxidants, and dietary plant polyphenols. Curr. Pharm. Biotechnol. 11, 837–848 (2010)
H.J. Park, M.J. Kim, E. Ha, J.H. Chung, Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedicine 15, 147–151 (2008)
A. Ghorban, M. Nazari, M. Jeddi-Tehrani, H. Zand, The citrus flavonoid hesperidin induces p53 and inhibits NF-κB activation in order to trigger apoptosis in NALM-6 cells: involvement of PPARγ-dependent mechanism. Eur. J. Nutr. 51, 39–46 (2012)
M. Nazari, A. Ghorbani, A. Hekmat-Doost, M. Jeddi-Tehrani, H. Zand, Inactivation of nuclear factor-κB by citrus flavanone hesperidin contributes to apoptosis and chemo-sensitizing effect in Ramos cells. Eur. J. Pharmacol. 650, 526–533 (2011)
K. Mohankumar, S. Pajaniradje, S. Sridharan, V.K. Singh, L. Ronsard, A.C. Banerjea, B.C. Selvanesan, M.S. Coumar, L. Periyasamy, R. Rajagopalan, Apoptosis induction by an analog of curcumin (BDMC-A) in human laryngeal carcinoma cells through intrinsic and extrinsic pathways. Cell. Oncol. 37, 439–454 (2014)
S. Aranganathan, N. Nalini, Efficacy of the potential chemopreventive agent, hesperetin [citrus flavanone], on 1,2-dimethylhydrazine induced colon carcinogenesis. Food Chem. Toxicol. 47, 2594–2600 (2009)
C.J. Lee, L. Wilson, M.A. Jordan, V. Nguyen, J. Tang, G. Smiyun, Hesperidin suppressed proliferations of both human breast cancer and androgen-dependent prostate cancer cells. Phytother. Res. Suppl 1, S15–S19 (2010)
G. Saiprasad, P. Chitra, R. Manikandan, G. Sudhandiran, Hesperidin induces apoptosis and triggers autophagic markers through inhibition of Aurora-A mediated phosphoinositide-3-kinase/Akt/mammalian target of rapamycin and glycogen synthase kinase-3 beta signalling cascades in experimental colon carcinogenesis. Eur. J. Cancer 50, 2489–2507 (2014)
S. Yumnam, HS. Park, MK. Kim, A. Nagappan, GE. et al, Hesperidin induces paraptosis like cell death in hepatoblatoma, HepG2 cells: involvement of ERK1/2 MAPK. PLoS One 30;9(6):e101321 (2014)
Acknowledgments
This work was funded by Istanbul University Scientific Research Project number 9205. We would like to thank Mr. David Chapman for editing the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

Supplemental Fig. 7
FGF signaling network. FGF network of 50 μM hesperidin-stimulated genes in A549 (a), NCI-H358(b) and MRC-5(c) cells. Ingenuity pathways analysis (IPA) software was used to identify genes involved in the FGF signaling that were differentially expressed in A549, NCI-H358 and MRC-5 cells. Genes labeled in red and green were identified as up- and down-regulated, respectively, whereas other genes were identified on the basis of the network analysis. (GIF 32 kb)
Fig. 7
High Resolution Image (TIFF 1736 kb)

Supplemental Fig. 8
NF-κB signaling network. NF-κB network of 50 μM hesperidin-stimulated genes in NCI-H358 cells. Ingenuity pathways analysis (IPA) software was used to identify genes involved in the NF-κB signaling that were differentially expressed in NCI-H358 cells. Genes labeled in red and green were identified as up- and down-regulated, respectively, whereas other genes were identified on the basis of the network analysis. (JPEG 131 kb)
ESM 1
(DOCX 14 kb)
ESM 2
(DOCX 14 kb)
ESM 3
(DOC 31 kb)
ESM 4
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Birsu Cincin, Z., Unlu, M., Kiran, B. et al. Anti-proliferative, apoptotic and signal transduction effects of hesperidin in non-small cell lung cancer cells. Cell Oncol. 38, 195–204 (2015). https://doi.org/10.1007/s13402-015-0222-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-015-0222-z