Abstract
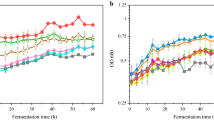
In order to achieve strain improvement and reduction of fermentation medium cost, atmospheric and room temperature plasma (ARTP) mutagenesis and microbial microdroplet culture (MMC) high-throughput screening were used to obtain high-yield Aurantiochytrium sp. strain. Meanwhile, low-cost food waste liquid was added into culture medium to reduce cost. The results showed that the optimal treatment time of ARTP mutagenesis for Aurantiochytrium sp. was 25 s, and 20 mutant strains were screened out by MMC to investigate their performance. The most significant increase in DHA yield was mutant Aurantiochytrium sp. MA20, whose biomass, lipid, and DHA yield were 29.0 ± 0.24, 15.8 ± 0.11, and 9.22 ± 0.37 g/L and 5.77, 16.9, and 83.2% higher than those of original strain, respectively. Compared with the original strain, the DHA proportion in total fatty acids of Aurantiochytrium sp. MA20 increased from 37.2 to 58.3%, while the DHA proportion in biomass increased from 18.4 to 31.7%. The genetic stability for Aurantiochytrium sp. MA20 showed that the yield of biomass, lipid, and DHA of the 50th generation were 29.1 ± 0.13, 16.2 ± 0.31, and 9.47 ± 0.26 g/L, respectively, which was no significant difference between original strain, indicating that the mutant MA20 could be inherited stably. Using food waste liquid to replace 100% yeast extract and 50% sea water of the fermentation medium, the DHA yield reached 9.54 g/L, which was equivalent to that of traditional medium, while the cost of medium was reduced by 45% that achieved the recycle of food waste resource.
Graphical abstract

Highlights
-
ARTP mutagenesis aided MMC screening which was used for Aurantiochytrium sp. breeding.
-
Optimal mutant strain obtained 9.22 g/L DHA yield and improved by 83.2%.
-
The ratio of DHA in total fatty acids of mutant MA20 increased from 37.2 to 58.3%.
-
Adding food waste liquid instead of yeast extract reduced 45% of the medium cost.






Similar content being viewed by others
References
Gupta A, Singh D, Byreddy AR, Thyagarajan T, Sonkar SP, Mathur AS et al (2016) Exploring omega-3 fatty acids, enzymes and biodiesel producing thraustochytrids from Australian and Indian marine biodiversity. Biotechnol J 11:345–355
Calder PC (2015) Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Bba-Mol Cell Biol L 1851:469–484
Xu X, Huang C, Xu Z, Xu H, Wang Z, Yu X (2020) The strategies to reduce cost and improve productivity in DHA production by Aurantiochytrium sp.: from biochemical to genetic respects. Appl Microbiol Biot 104:9433–9447
Finco AMDO, Mamani LDG, Carvalho JCD, de Melo Pereira GV, Thomaz-Soccol V, Soccol CR (2017) Technological trends and market perspectives for production of microbial oils rich in omega-3. Crit Rev Biotechnol 37:656–671
Tocher DR, Betancor MB, Sprague M, Olsen RE, Napier JA (2019) Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: bridging the gap between supply and demand. Nutrients 11:11010089
Colombo SM, Rodgers TFM, Diamond ML, Bazinet RP, Arts MT (2020) Projected declines in global DHA availability for human consumption as a result of global warming. Ambio 49:865–880
Lee SA, Whenham N, Bedford MR (2019) Review on docosahexaenoic acid in poultry and swine nutrition: consequence of enriched animal products on performance and health characteristics. Animal Nutrition 5:11–21
Alagawany M, Elnesr SS, Farag MR, Abd El-Hack ME, Khafaga AF, Taha AE et al (2019) Omega-3 and omega-6 fatty acids in poultry nutrition: effect on production performance and health. Animals 9:9080573
Zhang M, Chen C, You C, Chen B, Wang S, Li Y (2019) Effects of different dietary ratios of docosahexaenoic to eicosapentaenoic acid (DHA/EPA) on the growth, non-specific immune indices, tissue fatty acid compositions and expression of genes related to LC-PUFA biosynthesis in juvenile golden pompano Trachinotus ovatus. Aquaculture 505:488–495
Madeira MS, Cardoso C, Lopes PA, Coelho D, Afonso C, Bandarra NM et al (2017) Microalgae as feed ingredients for livestock production and meat quality: a review. Livest Sci 205:111–121
Lu Q, Li H, Xiao Y, Liu H (2021) A state-of-the-art review on the synthetic mechanisms, production technologies, and practical application of polyunsaturated fatty acids from microalgae. Algal Res 55:102281
Sun X, Geng L, Ren L, Ji X, Hao N, Chen K et al (2018) Influence of oxygen on the biosynthesis of polyunsaturated fatty acids in microalgae. Bioresource Technol 250:868–876
Park W, Moon M, Shin S, Cho JM, Suh WI, Chang YK et al (2018) Economical DHA (docosahexaenoic acid) production from Aurantiochytrium sp. KRS101 using orange peel extract and low cost nitrogen sources. Algal Res 29:71–79
Kothri M, Mavrommati M, Elazzazy AM, Baeshen MN, Moussa TAA, Aggelis G (2020) Microbial sources of polyunsaturated fatty acids (PUFAs) and the prospect of organic residues and wastes as growth media for PUFA-producing microorganisms. FEMS Microbiol Lett 367:028
Lopes Da Silva T, Moniz P, Silva C, Reis A (2019) The dark side of microalgae biotechnology: a heterotrophic biorefinery platform directed to ω-3 rich lipid production. Microorganisms 7:670
Ramos-Vega A, Rosales-Mendoza S, Bañuelos-Hernández B, Angulo C (2018) Prospects on the use of Schizochytrium sp. to develop oral vaccines. Front Microbiol 9:02506
Xie Y, Sen B, Wang G (2017) Mining terpenoids production and biosynthetic pathway in thraustochytrids. Bioresource Technol 244:1269–1280
Fossier Marchan L, Lee Chang KJ, Nichols PD, Mitchell WJ, Polglase JL, Gutierrez T (2018) Taxonomy, ecology and biotechnological applications of thraustochytrids: a review. Biotechnol Adv 36:26–46
Orozco Colonia BS, De Melo V, Pereira G, Soccol CR (2020) Omega-3 microbial oils from marine thraustochytrids as a sustainable and technological solution: a review and patent landscape. Trends Food Sci Tech 99:244–256
Ratledge C (2012) Omega-3 biotechnology: errors and omissions. Biotechnol Adv 30:1746–1747
Venkata Subhash G, Rajvanshi M, Navish Kumar B, Govindachary S, Prasad V, Dasgupta S (2017) Carbon streaming in microalgae: extraction and analysis methods for high value compounds. Bioresource Technol 244:1304–1316
Du F, Wang Y, Xu Y, Shi T, Liu W, Sun X et al (2021) Biotechnological production of lipid and terpenoid from thraustochytrids. Biotechnol Adv 48:107725
Arora N, Yen H, Philippidis GP (2020) Harnessing the power of mutagenesis and adaptive laboratory evolution for high lipid production by oleaginous microalgae and yeasts. Sustainability-Basel 12:5125
Sun X, Ren L, Bi Z, Ji X, Zhao Q, Jiang L et al (2018) Development of a cooperative two-factor adaptive-evolution method to enhance lipid production and prevent lipid peroxidation in Schizochytrium sp. Biotechnol Biofuels 11:1065–1074
Sun X, Ren L, Bi Z, Ji X, Zhao Q, Huang H (2018) Adaptive evolution of microalgae Schizochytrium sp. under high salinity stress to alleviate oxidative damage and improve lipid biosynthesis. Bioresource Technol 267:438–444
Fajardo C, De Donato M, Carrasco R, Martinez-Rodriguez G, Miguel Mancera J, Javier Fernandez-Acero F (2020) Advances and challenges in genetic engineering of microalgae. Rev Aquacul 12:365–381
Zeng W, Guo L, Xu S, Chen J, Zhou J (2020) High-throughput screening technology in industrial biotechnology. Trends Biotechnol 38:888–906
Ottenheim C, Nawrath M, Wu JC (2018) Microbial mutagenesis by atmospheric and room-temperature plasma (ARTP): the latest development. Bioresour Bioprocess 1–14
Cao S, Zhou X, Jin W, Wang F, Tu R, Han S et al (2017) Improving of lipid productivity of the oleaginous microalgae Chlorella pyrenoidosa via atmospheric and room temperature plasma (ARTP). Bioresource Technol 244:1400–1406
Fang M, Jin L, Zhang C, Tan Y, Jiang P, Ge N et al (2013) Rapid mutation of Spirulina platensis by a new mutagenesis system of atmospheric and room temperature plasmas (ARTP) and generation of a mutant library with diverse phenotypes. Plos One 1–12
Yu Q, Li Y, Wu B, Hu W, He M, Hu G (2020) Novel mutagenesis and screening technologies for food microorganisms: advances and prospects. Appl Microbiol Biot 104:1517–1531
Wang J, Jian X, Xing X, Zhang C, Fei Q (2020) Empowering a methanol-dependent Escherichia coli via adaptive evolution using a high-throughput microbial microdroplet culture system. Front Bioeng Biotechnol 8:570
Baig MB, Al-Zahrani KH, Schneider F, Straquadine GS, Mourad M (2019) Food waste posing a serious threat to sustainability in the Kingdom of Saudi Arabia - a systematic review. Saudi J Biol Sci 26:1743–1752
Du M, Liu X, Wang D, Yang Q, Duan A, Chen H et al (2021) Understanding the fate and impact of capsaicin in anaerobic co-digestion of food waste and waste activated sludge. Water Res 188:116539
Wang D, Yi N, Wang Y, Yang J, Fu Q, Liu X et al (2021) Triclosan degradation in sludge anaerobic fermentation and its impact on hydrogen production. Chem Eng J 421:129948
Patel A, Rova U, Christakopoulos P, Matsakas L (2020) Mining of squalene as a value-added byproduct from DHA producing marine thraustochytrid cultivated on food waste hydrolysate. Sci Total Environ 736:139691
Pleissner D, Lam WC, Sun Z, Lin CSK (2013) Food waste as nutrient source in heterotrophic microalgae cultivation. Bioresource Technol 137:139–146
Wang Q, Sun J, Liu S, Gao L, Zhou X, Wang D et al (2019) Free ammonia pretreatment improves anaerobic methane generation from algae. Water Res 162:269–275
Kube M, Spedding B, Gao L, Fan L, Roddick F (2020) Nutrient removal by alginate-immobilized Chlorella vulgaris: response to different wastewater matrices. J Chem Technol Biot 95:1790–1799
APHA (2012) Standard methods for the examination of water and wastewater. American Public Health Association AWWA (American Water Works Association), Washington, D.C., America
Xu H, Lin C, Shen Z, Gao L, Lin T, Tao H et al (2020) Molecular characteristics of dissolved organic nitrogen and its interaction with microbial communities in a prechlorinated raw water distribution system. Environ Sci Technol 54:1484–1492
Wang Q, Jin W, Zhou X, Zhang C, Gao S, Chen Y (2021) Enhancement of biodiesel-promising microalgae Chlorella pyrenoidosa growth using stimulants in municipal sewage. Biomass Conv Bioref 20:1204
Wang Q, Zhou X, Jin W, Zhang C, Liang Y, He Z et al (2021) Enhancing cultivation of biodiesel-promising microalgae Chlorella pyrenoidosa using plant hormones in municipal wastewater. Biomass Conv Bioref 21:1755
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Tu R, Jin W, Wang M, Han S, Abomohra AE, Wu W (2016) Improving of lipid productivity of the biodiesel promising green microalga Chlorella pyrenoidosa via low-energy ion implantation. J Appl Phycol 28:2159–2166
Han S, Jin W, Abomohra AE, Tu R, Zhou X, He Z et al (2019) Municipal wastewater enriched with trace metals for enhanced lipid production of the biodiesel-promising microalga Scenedesmus obliquus. Bioenerg Res 12:1127–1133
Guo D, Tong L, Ji X, Ren L, Ding Q (2020) Development of a strategy to improve the stability of culture environment for docosahexaenoic acid fermentation by Schizochytrium sp. Appl Biochem Biotech 20:03298
Ju J, Ko D, Heo S, Lee J, Kim Y, Lee B et al (2020) Regulation of lipid accumulation using nitrogen for microalgae lipid production in Schizochytrium sp. ABC101. Renew Energ 153:580–587
Hu F, Clevenger AL, Zheng P, Huang Q, Wang Z (2020) Low-temperature effects on docosahexaenoic acid biosynthesis in Schizochytrium sp. TIO01 and its proposed underlying mechanism. Biotechnol Biofuels 13:01811
Morabito C, Bournaud C, Maes C, Schuler M, AieseCigliano R, Dellero Y et al (2019) The lipid metabolism in thraustochytrids. Prog Lipid Res 76:101007
Li Y, Xu H, He C, Shen Z, Chen W, Gao L et al (2019) Transformation and fate of dissolved organic nitrogen in drinking water supply system: a full scale case study from Yixing, China. Sci Total Environ 673:435–444
Liang L, Zheng X, Fan W, Chen D, Huang Z, Peng J et al (2020) Genome and transcriptome analyses provide insight into the omega-3 long-chain polyunsaturated fatty acids biosynthesis of Schizochytrium limacinum SR21. Front Microbiol 11:687
Liu L, Hu Z, Li S, Yang H, Li S, Lv C et al (2020) Comparative transcriptomic analysis uncovers genes responsible for the DHA enhancement in the mutant Aurantiochytrium sp. Microorganisms 8:529
Park H, Kwak M, Seo J, Ju J, Heo S, Park S et al (2018) Enhanced production of carotenoids using a thraustochytrid microalgal strain containing high levels of docosahexaenoic acid-rich oil. Bioproc Biosyst Eng 41:1355–1370
Fu J, Chen T, Lu H, Lin Y, Xie X, Tian H et al (2016) Enhancement of docosahexaenoic acid production by low-energy ion implantation coupled with screening method based on Sudan black B staining in Schizochytrium sp. Bioresource Technol 221:405–411
Cheng Y, Sun Z, Cui G, Song X, Cui Q (2016) A new strategy for strain improvement of Aurantiochytrium sp based on heavy-ions mutagenesis and synergistic effects of cold stress and inhibitors of enoyl-ACP reductase. Enzyme Microb Tech 93–94:182–190
Li D, Zhang K, Chen L, Ding M, Zhao M, Chen S (2017) Selection of Schizochytrium limacinum mutants based on butanol tolerance. Electron J Biotechn 30:58–63
Lian M, Huang H, Ren L, Ji X, Zhu J, Jin L (2010) Increase of docosahexaenoic acid production by Schizochytrium sp through mutagenesis and enzyme assay. Appl Biochem Biotech 162:935–941
Zhang X, Zhang X, Li H, Wang L, Zhang C, Xing X et al (2014) Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool. Appl Microbiol Biot 98:5387–5396
Wang Q, Han W, Jin W, Gao S, Zhou X (2021) Docosahexaenoic acid production by Schizochytrium sp.: review and prospect. Food Biotechnol 35:111–135
Funding
This work was supported by the National Natural Science Foundation of China (No.51878215), the Key Areas Research and Development Program of Guangdong Province, China (2019B110205001), the Natural Science Foundation of Shenzhen (No. GXWD20201230155427003-20200821174135002), and the Demonstration Project for Marine Economic Development in Shenzhen to Dr. Zhangli Hu, China’s State Oceanic Administration, and Shenzhen Science and Technology Innovation Project (KJYY20171011144235970).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Q., Jin, W., Han, W. et al. Enhancement of DHA production from Aurantiochytrium sp. by atmospheric and room temperature plasma mutagenesis aided with microbial microdroplet culture screening. Biomass Conv. Bioref. 13, 16807–16818 (2023). https://doi.org/10.1007/s13399-021-02147-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02147-9