Abstract
The virology of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and the human immune response to the virus are under vigorous investigation. There are now several reports describing neurological symptoms in individuals who develop coronavirus disease 2019 (COVID-19), the syndrome associated with SARS-CoV-2 infection. The prevalence, incidence, and clinical course of these symptoms will become clearer in the coming months and years through epidemiological studies. However, the long-term neurological and cognitive consequence of SARS-CoV-2 infection will remain conjectural for some time and will likely require the creation of cohort studies that include uninfected individuals. Considering the early evidence for neurological involvement in COVID-19 it may prove helpful to compare SARS-CoV-2 with another endemic and neurovirulent virus, human immunodeficiency virus-1 (HIV-1), when designing such cohort studies and when making predictions about neuropsychological outcomes. In this paper, similarities and differences between SARS-CoV-2 and HIV-1 are reviewed, including routes of neuroinvasion, putative mechanisms of neurovirulence, and factors involved in possible long-term neuropsychological sequelae. Application of the knowledge gained from over three decades of neuroHIV research is discussed, with a focus on alerting researchers and clinicians to the challenges in determining the cause of neurocognitive deficits among long-term survivors.
Similar content being viewed by others
COVID-19: neurological symptoms and outcomes
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the associated syndrome, coronavirus disease 2019 (COVID-19), first gained attention in December of 2019 in the city of Wuhan, China (World Health Organization: Naming the coronavirus disease (COVID-19) and the virus that causes it 2020). Transmission to humans was zoonotic, apparently originating in bats (Zhou et al. 2020), possibly via pangolins (Lam et al. 2020) and/or snakes (Ji et al. 2020). Herein, we use the terms SARS-CoV-2 and COVID-19 to describe the virus and its associated clinical syndrome, respectively.
All studies of neurological outcomes have occurred in individuals diagnosed with COVID-19 and have consisted of case reports and case series. There are, as of yet, no studies of the neuropsychological sequelae of SARS-CoV-2 infection or COVID-19 illness, although helpful speculative articles have been published (Condie 2020) and others are forthcoming. The largest published study of neurological symptoms in laboratory-confirmed SARS-CoV-2-infected cases comes from Wuhan, China (Mao et al. 2020). In that case series, 36.4% of 214 patients developed neurological symptoms that included headache, dizziness, and (less commonly) mental status change and paresthesia. It is important to point out that 41% of the sample from the study had severe illness, and it was the more severely affected patients who exhibited the neurological symptoms, suggesting that neurological symptoms could have been related to systemic factors or exacerbation of preexisting medical conditions. In another case series of 58 COVID-19 patients (median age 63 years) admitted to a French hospital due to acute respiratory distress syndrome (ARDS), 84% had neurological symptoms at some point during their hospitalization, 14% of whom presented with neurological symptoms upon admission (Helms et al. 2020). Corticospinal tract symptoms were observed in 67%, and 36% had dysexecutive syndrome (e.g., inattention and disorientation). Of those who underwent magnetic resonance imaging (MRI) of the brain, 62% had leptomeningeal enhancement and 23% had cerebral ischemic stroke. All eleven patients who underwent perfusion imaging had bilateral frontotemporal hypoperfusion; however, only one of eight patients who received EEG testing had nonspecific abnormal findings. It is unclear whether the patients with known preexisting neurological disorders were among those showing symptoms. A study of 43 COVID-19 patients in England (Paterson et al. 2020) suggested five major categories of neurological involvement: (i) encephalopathies (n = 10), (ii) inflammatory CNS syndromes (n = 12), (iii) ischemic strokes (n = 8), (iv) peripheral neurological disorders (n = 8) primarily consisting of Guillain-Barré syndrome, and (v) patients with CNS disorders who did not fit into these categories (n = 5). Finally, it is notable that other case series have not reported a significant incidence of neurological symptoms. For example, among 41 COVID-19 patients in China, only headaches were reported among 8% of the sample (Huang et al. 2020). Also, two larger case series currently in pre-print, one of 20,662 patients in China (Gu 2020) and the other of 1000 patients in New York City (Argenziano et al. 2020), do not describe prominent neurological symptoms, with the possible exception of headache. Whether these discrepant findings are due to true differences in the populations or the syndromes described or to a lack of standardized data and/or symptom collection methods is unclear.
Hyposmia/anosmia and hypogeusia/dysgeusia have received significant media attention. Such symptoms were reported in the first case series described above (Mao et al. 2020) and in a case series from Italy (Giacomelli et al. 2020). Whether or not these symptoms are truly neurological in nature is uncertain at this time. For example, a case study from France indicated that bilateral obstructive inflammation of olfactory clefts impaired olfactory function by preventing odorant molecules from reaching the olfactory epithelium (Eliezer et al. 2020), suggesting that loss of smell or taste may not have a neurological cause, at least not in all cases. Furthermore, a gene expression study indicates that it is the infection of non-neuronal cell types that leads to anosmia and aberrant odor perception in COVID-19 patients (Brann et al. 2020). Conversely, while anosmia is known to occur in many upper respiratory tract infections, many patients with COVID-19 lose smell despite absence of congestion (Pleasure et al. 2020).
A steady stream of case reports has described severe acute neurologic conditions in a minority of COVID-19 patients, some of whom were relatively young. One such study from Japan described a 24-year-old man who developed meningitis and encephalitis. MRI findings from that case included hyperintensity along the wall of the right lateral ventricle and hyperintense signal changes in the right mesial temporal lobe and hippocampus (Moriguchi et al. 2020). Another case report described acute necrotizing hemorrhagic encephalopathy in a 50+-year-old female with laboratory-verified COVID-19 (Poyiadji et al. 2020). Head CT revealed symmetric hypoattenuation within the medial thalamic nuclei bilaterally, whereas brain MRI revealed hemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions. Her premorbid medical history was not described. Five younger (< 50 years old) patients presented with large-vessel stroke within a 2-week period at a New York City hospital, where historically an average of only 0.73 patients under 50 were treated for the condition during the same time frame over the previous 12 months (Oxley et al. 2020). A similar case series, describing six patients with stroke under the age of 55, was recently reported from Iran (Ashrafi et al. 2020).
In summary, these early case reports suggest that a minority of COVID-19 cases have neurological symptoms ranging in severity from very mild (e.g., headache and loss of smell/taste) to severe (e.g., encephalitis), and that symptoms can occur before or after other “core” symptoms of COVID-19 (Mao et al. 2020). However, the meaning of these observations is unclear, as the studies described above did not include control groups consisting of uninfected patients with similarly severe respiratory or other symptoms or those who had undergone similar medical procedures (e.g., ventilation and sedation) for other conditions. Additionally, it is unclear if the discrepancy of findings in case series is due to differences in data collection methods and whether the patients described in the aforementioned studies were tested for other potentially neuroinvasive viruses that can lead to similar neurological conditions as those reported for COVID-19, such as influenza A (Ozkale et al. 2012; Toovey 2008; Wang et al. 2010).
Potential mechanisms of central nervous system involvement in SARS-CoV-2 infection
Previously identified human coronaviruses (HCoV) are neuroinvasive and, as the evidence reviewed below suggests, neurovirulent. Initial evidence suggests that SARS-CoV-2 is also neuroinvasive, although its neurovirulence will take some time to determine. Below, we review and discuss potential acute and long-term neurological and neuropsychological sequelae due to direct and indirect routes of pathogenicity. Other reviews are also available (Sepehrinezhad et al. 2020).
Direct causes
The aforementioned case studies suggest that SARS-CoV-2 is both neuroinvasive and neurovirulent. Furthermore, research findings of other viruses, including HCoVs, raise the possibility that it may persist in the central nervous system (CNS) following systemic clearance. As described in a recent review, coronaviruses can enter the CNS via two routes (Desforges et al. 2019). The first is the hematogenous route, in which HCoVs enter the circulatory system via the human airway epithelium (Dijkman et al. 2013) and then infect epithelial cells of blood–brain barrier (BBB) or blood–cerebrospinal fluid barrier in the choroid plexus. HCoVs can also infect myeloid cells, which then shuttle it into CNS (Gu et al. 2005; Desforges et al. 2007; Collins 2002), a route favored by HIV-1 (Kim et al. 2003; Argyris et al. 2007; Atluri et al. 2015; Wang et al. 2008). In both cases, HCoVs enter the cells by first binding to a the ACE2 receptor, after which they enter endosomes and fuse the viral and lysosomal membranes (Shang et al. 2020). The second is the neuronal retrograde route, in which viruses exploit periphery nerves and axonal transport mechanisms to gain entry to CNS (Dahm et al. 2016; Desforges et al. 2014a). This could occur via several possible cranial nerves, including olfactory (Mori 2015), trigeminal (Lochhead et al. 2019; Lochhead and Thorne 2012), and vagus nerves (Matsuda et al. 2004; Park et al. 2002). HCoV-OC43, a coronavirus that infects humans and cattle with generally mild symptoms, uses this neuron-to-neuron route (Dube et al. 2018). In addition, both SARS-CoV-1 and the Middle East respiratory syndrome coronavirus (MERS-CoV) can also gain access to the CNS via peripheral nerve terminals and subsequent synapse-connected pathways (Li et al. 2012, 2013; Andries and Pensaert 1980). In transgenic mice, MERS-CoV then spreads to the brainstem, thalamus, and other brain regions (Li et al. 2016a). There is evidence that SARS-CoV-2 also has the potential to be neuroinvasive. SARS-CoV-2 enters angiotensin-converting enzyme 2 (ACE2)-expressing cells, which are found in the airway epithelia, lungs, vascular endothelia, kidney, and small intestine (Li et al. 2020). ACE2 is also expressed in the mouse and rat brains, in particular the brainstem regions that control cardiovascular functioning (Gowrisankar and Clark 2016; Xia and Lazartigues 2010), suggesting a direct role of the virus in ARDS (Li et al. 2020).
While most, if not all, HCoVs are neuroinvasive, only some appear neurovirulent (Li et al. 2016a; Glass et al. 2004; Talbot et al. 1993). SARS-CoV-1, HCoV-OC43, and HCoV-229E (a coronavirus that infects bats and humans, with generally mild symptoms to the latter) are known to be neurotropic in humans (Xu et al. 2005; Arbour et al. 1999, 2000). SARS-CoV-1 causes neuronal death in the absence of encephalitis in human ACE2 transgenic mice (Netland et al. 2008), and neuropathological findings from humans who died of SARS, the syndrome caused by SARS-CoV-1, included cerebral edema, meningeal vasodilation, ischemic changes of neurons, and demyelination (Gu et al. 2005; Xu et al. 2005; Netland et al. 2008). HCoV-OC43 infection of mouse CNS induces glutamate excitotoxicity with subsequent neuronal damage and disruption of glutamate homeostasis, with the downstream effect being limb paralysis and possible demyelination (Desforges et al. 2019). HCoV-OC43 has also been linked to encephalitis in humans (Morfopoulou et al. 2016). Evidence that MERS-CoV is neurovirulent includes case reports of psychosis and seizures (Saad et al. 2014), as well as mental status changes, paralysis, ischemic stroke, Guillian-Barre syndrome, and neuropathy that arise 2–3 weeks after resolution of respiratory symptoms (Kim et al. 2017).
When assessing the possible neurovirulence of SARS-CoV-2, one could consider genetic similarities with other HCoVs. SARS-CoV-2 and SARS-CoV-1 are 79% genetically homologous (Lu et al. 2020). A mutation in a gene that produces SARS-CoV-2 spike protein gives the virus an affinity for ACE2 that is at least 10× greater than SARS-CoV-1 (Wrapp et al. 2020), likely explaining in part the former’s greater infectivity. During the 2002–2003 SARS outbreak, a variety of neurological conditions appeared 3–4 weeks into the course of the illness in a small number of patients, including polyneuropathy, encephalitis, and aortic ischemic stroke (Tsai et al. 2005). This seems to differ from SARS-CoV-2, in which some neurological symptoms appear earlier in the course of illness (Mao et al. 2020). Whether this is due to the difference in ACE2 affinity or other functional differences will be the subject of future studies. Infection with the Middle East Respiratory Syndrome (MERS) coronavirus (MERS-CoV) led to the deadliest syndrome in 2012. As with SARS-CoV-1 and 2, MERS-CoV originated in bats (Memish et al. 2013), with the dromedary serving as the vector to humans. However, it is only 50% genetically homologous to SARS-CoV-2 (Lu et al. 2020).
Finally, the chronic presence of SARS-CoV-2 in the brain could conceivably result in long-term neurological and neuropsychological sequelae. Several other viruses exert chronic deleterious effects in human CNS. The most well-studied example is HIV-1 infection, as discussed in detail below. Other examples include human herpes viruses, which have been associated with later risk of Alzheimer’s disease, multiple sclerosis (MS), and other neurodegenerative disorders (Leibovitch and Jacobson 2018; Itzhaki et al. 2004; Ludlow et al. 2016; Majde 2010), and influenza A, which is associated with later risk for Parkinson’s disease (Jang et al. 2009). The long-term persistence of HCoVs in the CNS and their potential to cause delayed neurologic dysfunction (Arbour et al. 1999; Cristallo et al. 1997; Fazzini et al. 1992; Stewart et al. 1992; Murray et al. 1992; Johnson-Lussenburg and Zheng 1987) may be underestimated. Several studies report putative links between HCoV in the human neurologic disorders, including encephalitis (Morfopoulou et al. 2016), multiple sclerosis (Cristallo et al. 1997; Stewart et al. 1992), Parkinson’s disease (Fazzini et al. 1992), and acute demyelinating encephalomyelitis (Yeh et al. 2004). Both HCoV-229E and HCoV-OC43 have been found in the brains of deceased MS patients (Talbot et al. 1993; Arbour et al. 2000; Murray et al. 1992; Burks et al. 1980) and those who had encephalomyelitis (Yeh et al. 2004; Li et al. 2016b). However, it is important to consider that the high prevalence of some HCoVs in human tissue makes it difficult to interpret such findings (Desforges et al. 2014b).
Indirect causes
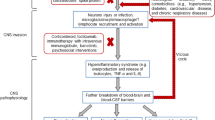
Even without considering its potential for neurovirulence, SARS-CoV-2 infection may lead to poor neurological outcomes via indirect routes. There have been recent reports in the media about high stroke rates in relatively young patients being treated for COVID-19, but published findings are scant. A case series described three COVID-19 patients aged 65–70 in Wuhan, China, who developed coagulopathy and antiphospholipid antibodies with subsequent multiple cerebral infarcts, damaging a wide range of brain regions (Zhang et al. 2020). All had history of hypertension and other medical illnesses. In addition, the reported suppressed levels of platelets and elevated levels of D-dimer (a protein fragment measured in the blood to diagnose thrombosis, embolism, and coagulation) (Wang et al. 2020) may make patients with severe COVID-19 more prone to cerebrovascular accidents. This notion is supported by the findings of a retrospective study of COVID-19 patients seen in New York City. In that study, patients who had a brain MRI with evidence leukoencephalopathy and/or cerebral microbleeds had high peak D-dimer levels and lower nadir platelet counts compared with those who did not have findings of such pathology (Agarwal et al. 2020). Those patients who develop acute respiratory distress syndrome (ARDS) are at risk of cerebral hypoxemia (Hopkins et al. 2006). Many of those with ARDS are intubated and treated with mechanical ventilators, a procedure with substantial risks (Hoesch et al. 2012). Indeed, a case series of eighteen deceased COVID-19 patients aged 53–75 who underwent neuropathological examination revealed only signs of hypoxia in brain tissue (Solomon et al. 2020). Eleven had received mechanical ventilation before death. Also notable is that virus was detected at low levels in only five patients.
The reported “cytokine storms” reported in severe COVID-19 cases (Mehta et al. 2020) can lead to multiple organ damage, leading to renal and hepatic liver dysfunction and cardiac dysfunction (South et al. 2020), all of which can have adverse effects on cognitive functioning (Bennett and Sauve 2003; Patel et al. 2015; Kurella Tamura et al. 2011). Indeed, this acute inflammatory state can also lead to CNS damage (Clark and Vissel 2017). Whether or not cytokine-driven neuroinflammation might also be present in infected individuals with more mild symptoms might also be considered in future studies. Finally, some have proposed that CNS-related autoimmune disorders could arise post-SARS-CoV-2 infection (Troyer et al. 2020) via “molecular mimicry” (Rose 2017), as has been reported in SARS-CoV-1 and MERS-CoV infections (Kim et al. 2017; Tsai et al. 2005).
In summary, there is ample evidence from COVID-19 case reports and studies of other HCoVs that SARS-CoV-2 is both neuroinvasive and neurovirulent, possibly via different routes. Furthermore, poor neurological outcomes can result from indirect causes linked to systemic infection and aggressive treatment of COVID-19, which is similar to HIV-1.
HIV-1: summary of neurological and neuropsychological symptoms and sequelae
A dementia syndrome associated with HIV-1 was first systematically described and termed AIDS dementia complex in 1986 (Navia et al. 1986). For the next 10 years, the prevalence of what came to be more commonly referred to as HIV-associated dementia was about 16%(McArthur et al. 1993) and typically indicated advanced immune dysfunction and a poor prognosis. After combined or highly active antiretroviral therapy (cART or HAART) became widely available in 1996, the prevalence of HIV-associated dementia dropped to less than 5% (Dore et al. 2003; Robertson et al. 2007; Sacktor et al. 2002). However, a more chronic and less severe form of cognitive impairment became evident, leading to updated research criteria published in 2007 that capture the full spectrum of neuropsychological deficits thought to be due to HIV (Antinori et al. 2007). The term for this broad classification that captures the full severity range of cognitive impairment, from mild deficits without noticeable impact on day-to-day functioning to debilitating dementia, is HIV-associated neurocognitive disorders or HAND. The nature of cognitive deficits varies widely and has also changed over time as HIV-1 infection has become more chronic in nature (Heaton et al. 2011). Estimates of the current prevalence of HAND vary considerably, with between 15 and 84% of infected individuals meeting criteria at any one time (Heaton et al. 2011; Cysique et al. 2004; Becker et al. 2004; Bonnet et al. 2013; Simioni et al. 2010; Saylor et al. 2016). A majority of HAND diagnoses are mild, termed asymptomatic neurocognitive impairment (ANI) according to current research criteria (Antinori et al. 2007). However, the inclusion of ANI in current diagnostic schema may have had the unintended consequence of high rates of false positive diagnoses due to the low threshold for cognitive impairment, thereby inflating HAND prevalence estimates (Gisslen et al. 2011; Meyer et al. 2013). Indeed, a significant percentage of healthy HIV-uninfected individuals with no known neurologic or psychiatric illness would meet criteria for ANI, save for the fact that they are not HIV infected (Meyer et al. 2013; Schretlen et al. 2003; Palmer et al. 1998; Schretlen et al. 2008; Binder et al. 2009). Indeed, two recent studies have attempted to deal with the problem of high false discovery rates using the method of multivariate normative comparison (Wang et al. 2019a; Su et al. 2015). In both studies, the rate of cognitive impairment among HIV-infected individuals was almost identical to that of uninfected individuals, demonstrating that empirically defined thresholds for impairment may be a more reliable method.
Both in the pre-cART and current eras (i.e., since 1996), HIV encephalitis (HIVE) is considered to be a major neuropathological basis of HIV-associated dementia (McArthur et al. 1993; Moore et al. 2006a; Glass et al. 1995; Bell et al. 1998; Persidsky and Gendelman 2003; Everall et al. 2005; Letendre et al. 2011; Boven et al. 2000; Conant et al. 1998; Eugenin et al. 2006; Kraft-Terry et al. 2009). However, a vast majority of HAND cases present with milder symptoms (McArthur et al. 2005; Heaton et al. n.d.) and do not have neuropathological findings consistent with HIVE (Everall et al. 2009). Accumulating evidence suggests that for the vast majority of HAND cases that are mild-to-moderate in severity, cognitive impairment is due largely to synaptodendritic dysfunction driven by chronic CNS inflammation (Glass et al. 1995; Persidsky and Gendelman 2003; Kraft-Terry et al. 2009; Everall et al. 2009; Moore et al. 2006b; Levine et al. 2015; Crews et al. 2008; Guha et al. 2018). As with the direct and indirect mechanisms of CNS damage proposed for SARS-CoV-2 described above, the neuropathogenesis of HAND likely has direct and indirect routes. In the direct route, HIV-1 in brain macrophages and other cells releases viral proteins that harm nearby neurons and other cells (Glass et al. 1995; Kraft-Terry et al. 2009; Kedzierska and Crowe 2002; Adle-Biassette et al. 1999; Lindl et al. 2007; Kaul and Lipton 2006), and cross-talk between neurons and microglia can also drive synaptodendritic dysfunction (Alvarez-Carbonell et al. 2019). In the indirect route, macrophage proliferation, microglial activation, astroglial activation, and dysregulated cytokine expression and production (Glass et al. 1995; Bell et al. 1998; Persidsky and Gendelman 2003; Everall et al. 2005; Letendre et al. 2011; Boven et al. 2000; Conant et al. 1998; Eugenin et al. 2006; Kraft-Terry et al. 2009) drive inflammation, resulting in synaptodendritic dysfunction. Indeed, increased migration across the blood–brain barrier of monocytes (Pulliam et al. 1997; Ellery et al. 2007) driven both by chemokine gradients originating in the CNS and from a peripheral immune response (Kraft-Terry et al. 2009; Peluso et al. 1985; Ancuta et al. 2004) is thought to be a major factor underlying HAND (Boven 2000).
SARS-CoV-2 and HIV-1: differences in virology and host immune response
The virology of HIV-1 differs substantially from coronaviruses such as SARS-CoV-2. HIV-1 is a retrovirus whose RNAgenome is reverse-transcribed into double-stranded DNA and integrated into the cellular DNA, where it can remain hidden from the host’s immune surveillance in a latent state for 8–10 years, during which time the host remains symptom free (Siliciano and Greene 2011). Once activated, the integrated HIV-1 DNA uses the host’s replication mechanisms to create additional RNA genomes and viral proteins, which then exit the cell to propagate the infection, much as other viruses including HCoVs.
HIV-1 targets cells of the immune system, specifically CD4+ helper T cells (Coakley et al. 2005), monocytes (Kedzierska and Crowe 2002), macrophages (Kedzierska and Crowe 2002), and dendritic cells (Cunningham et al. 2010) via binding to CD4 receptor and CCR5 co-receptor, although some strains use CXCR4 as the co-receptors for viral entry (Chan et al. 1997; Arrildt et al. 2012). The body’s adaptive immunity defenses are largely ineffective against HIV-1. Cell-mediated immunity driven by T cells is largely disabled because of the virus’s predilection for those cells (Coakley et al. 2005). Furthermore, antibodies produced by B-cells are not effective against HIV-1. As such, without treatment with a combination of antiretroviral medications, the host becomes vulnerable to opportunistic infections and the eventual development of acquired immunodeficiency syndrome (AIDS). Virus-harboring macrophages move via chemotaxis to seed other body regions with the virus, including the CNS (Kraft-Terry et al. 2009; Peluso et al. 1985; Ancuta et al. 2004) which serves as a sanctuary for the virus. In the CNS, HIV-1 is largely safe from immunosurveillance, infecting microglia which serve as a primary reservoir of the virus (Wallet et al. 2019). In the case of SARS-CoV-2, the virus binds to the spike protein on the surface of epithelial cells, enters endosomes, and is then dispersed into the cytoplasm when the viral and lysosomal membranes fuse (Shang et al. 2020). It is unclear whether SARS-CoV-2 will also be able to persist in the brain. One proposed mechanism linking other HCoV infections with later neurologic disease is chronic infection of oligodendrocytes and glial cells (Arbour et al. 1999; Bender and Weiss 2010), perhaps due to the lower immunosurveillance in the brain that makes it a sanctuary for HIV-1. If this is also the case for SARS-CoV-2, our current understanding of antiviral medication penetrance into the CNS (Letendre et al. 2008), as well as innovative methods to eradicate viruses from the brain (Nowacek et al. 2010), will be useful.
That HIV-1 is a retrovirus, along with its high mutation rate, makes it almost impossible to eradicate from host cells. Only two individuals are known to have been completely cleared of HIV-1 following allogeneic hemopoietic stem cell transplantation from a donor homozygous for the delta-32 allele on the CCR5 gene (Gupta et al. 2020; Hutter et al. 2009). While there is no evidence of HIV-1 RNA in cerebrospinal fluid, it remains unknown whether HIV-1 persists in the brains of these individuals, who are still living.
It remains uncertain if lasting adaptive immunity to SARS-CoV-2 will be possible. Based on human immune response to other coronaviruses, at least temporary immunity is expected and illness from later infections may be less severe (Channappanavar et al. 2014). As with HIV-1, infectability and the host immune response may vary according to host genotype, although further study is required to validate these findings (Stawiski et al. 2020). For example, mutations in the ACE2 gene could conceivably influence infectivity and symptom type/severity. In the case of HIV-1, discovering that the CCR5 was a co-receptor (Choe et al. 1996) led to the development of a new class of antiviral medication (Imamura et al. 2004). However, the application of host genetics to HAND has been less successful (Kallianpur and Levine 2014), possibly owing to poor diagnostic reliability (Woods et al. 2004).
Finally, in regard to maladaptive immune responses, the “cytokine storms” described in COVID-19 patients that are responsible for severe organ damage and mortality are sometimes seen during the acute phase of HIV-1 infection, but in a less severe form (Erdmann and Heath 2019). However, upon initiation of antiviral therapy, a minority of HIV-infected patients can develop immune reconstitution inflammatory syndrome (Manzardo et al. 2015), which can result in significant morbidity and even death.
In summary, while there are fundamental differences in the virological and immune response to SARS-CoV-2 and HIV-1, their shared potential for CNS persistence and neurovirulence could make current knowledge of neuroHIV helpful for devising treatments and designing studies of the neuropsychological consequences of SARS-CoV-2 infection.
Considering long-term neuropsychological outcomes in SARS-CoV-2 infection: why comorbidities matter
Assuming that SARS-CoV-2 like other HCoVs can have a lasting presence in the CNS (Talbot et al. 1993; Arbour et al. 1999, 2000; Murray et al. 1992; Burks et al. 1980), its potential to provoke a chronic neuroinflammatory immune response similar to HIV-1 should be considered in the pathogenesis of neuropsychological deficits. Furthermore, long-term tracking of survivors will determine whether CNS exposure to the virus increases risk of later neurodegenerative illness (Arbour et al. 1999; Kim et al. 2017; Tsai et al. 2005; Cristallo et al. 1997; Fazzini et al. 1992; Stewart et al. 1992; Murray et al. 1992; Johnson-Lussenburg and Zheng 1987; Troyer et al. 2020; Rose 2017). In addition, based on findings from over 30 years of neuroHIV research, it is just as important to consider the various comorbidities and other factors that are more likely to affect neuropsychological functioning.
The prevalence of HAND may be overestimated. Large case-control studies consisting of only HIV+ participants (Robertson et al. 2007; Antinori et al. 2007; Heaton et al. 2010, Heaton et al. 2015) or those that included mismatched HIV− participants (Heaton et al. 2011) generally point to the virus itself as the primary cause of neurocognitive and neurophysiological aberrations. However, cohort studies that include well-matched HIV-uninfected control participants indicate that, at least in generally cART-treated individuals, it is primarily medical and psychiatric comorbidities that underlie neurocognitive impairment (Sacktor et al. 2016; Vance et al. 2016), with a smaller percentage of neurocognitively impaired cases apparently due to HIV infection. Similarly, neurophysiological changes in those with HIV, as determined through magnetic resonance imaging, appear due largely to medical comorbidities, including hypertension, diabetes mellitus, higher body mass index, and elevated visceral fat (Lake et al. 2017; Wu et al. 2018). It is interesting to note that age-related medical comorbidities are more prevalent in the context of HIV (Silverberg et al. 2009, 2011; Womack et al. 2011; Kirk et al. 2013; Lucas et al. 2007; Desquilbet et al. 2011), possibly due to accelerated biological aging caused by the retrovirus (Horvath and Levine 2015; Gross et al. 2016; Rickabaugh et al. 2015). As described in the opening section, case studies to date point to indirect, medical-related causes for neuropsychological deficits in COVID-19 survivors, including cerebrovascular pathologies (Zhang et al. 2020; Wang et al. 2020), cerebral hypoxemia (Hopkins et al. 2006), and chronic illnesses resulting from organ damage cause by acute cytokine dysregulation (South et al. 2020; Bennett and Sauve 2003; Patel et al. 2015; Kurella Tamura et al. 2011; Clark and Vissel 2017). In addition, that COVID-19 presents as more severe in older individuals and those with medical conditions that are also risk factors for cognitive impairment will further complicate the clinical picture. More specifically, hypertension, diabetes, cancer, cardiovascular disease, and chronic respiratory illness are risk factors for severe COVID-19 and/or death (World Health Organization: Coronavirus disease (COVID-19) Pandemic n.d.) and also for neuropsychological deficits (Bennett and Sauve 2003; Reijmer et al. 2011; van den Berg et al. 2010; Novak and Hajjar 2010; Schou et al. 2012; Janelsins et al. 2018; Ahles and Root 2018). In addition, some have posited that ACE2 inhibitors used to treat hypertension and diabetes may increase the expression of ACE2, making cells more vulnerable to SARS-CoV-2 infection and, as a consequence, making those individuals more prone to severe COVID-19 (Nath 2020). Therefore, determination of later neuropsychological impairment in survivors of SARS-CoV-2 infection and COVID-19 illness will require consideration of these and other medical comorbidities.
Not surprisingly, HIV-1 infection is associated with greater risk of psychiatric illness, and this can exacerbate and complicate cognitive impairment (Rubin et al. 2019; Rubin and Maki 2019; Spies et al. 2018; Cysique and Brew 2019). Depression can result from the personal and psychosocial impacts of the disease, as well as biological effects of infection and treatment (Lu et al. 2019). Considering the relatively high fatality rate among individuals who become infected with SARS-CoV-2, as well as the emotional and financial devastation caused by the pandemic, psychological disorders such as post-traumatic stress disorder (PTSD) and depression should be considered primary diagnoses and contributing factors to neurocognitive impairment (Troyer et al. 2020). Indeed, early indications suggest higher prevalence of anxiety disorders such as PTSD stemming from either surviving infection with SARS-CoV-2 or serving as a frontline healthcare worker (Liu et al. 2020), as well as those living near the epicenter of the outbreak (Sun et al. 2020). This appears consistent with studies from the 2003 SARS and 2005 MERS outbreaks, which reported high rates of PTSD (Tam et al. 2004; Maunder et al. 2004; Hong et al. 2009; Kim et al. 2016). The impact of death due to COVID-19 may also have a more profound impact on loved ones. In fact, one very important difference between COVID-19 and HIV-1 infections is that in the terminal stages of the latter, a patient’s family member or close friend is able to be bedside to express final thoughts of love and comfort. However, with COVID-19, this is not the case, as loved ones have been prohibited from accompanying terminally ill patients because of the extremely high risk of infection via respiratory droplets.
Finally, in order to accurately delineate long-term neuropsychological outcomes of SARS-CoV-2 infection, cognitive testing of individuals with active symptoms (i.e., COVID-19) should be avoided, as studies have shown that even very mild upper respiratory viral infections can have acute effects on neuropsychological functioning (Smith 2012, 2013).
Approaches for studying the neuropsychological sequelae of SARS-CoV-2 infection
In order to characterize the long-term neuropsychological sequelae of SARS-CoV-2 infection and/or COVID-19, large and diverse cohort studies that include both infected (historically or actively) and never-infected individuals will be required. Such studies will allow for longitudinal characterization of cognitive functioning while considering comorbidities and other potential factors affecting such functioning (e.g., medication type, psychiatric and medical comorbidities). Such cohort studies will also likely capture a substantial number of seroconverters (i.e., those who become infected during their study participation). Large cohort studies of HIV-1, such as the Multicenter AIDS Cohort Study and the Women’s Interagency HIV Study, have contributed significantly to the understanding of the natural and treated history of HIV-1 infection, including neuropsychological outcomes with consideration of medical and other comorbidities (Sacktor et al. 2016; Becker et al. 2014; Levine et al. 2007; Levine et al. 2013; Maki et al. 2009; Meyer et al. 2014; Rubin et al. 2017) but have been limited to domestic participants.
Drawing from the history of neuroHIV research, in which the Western research community either did not recognize or failed to appreciate until the mid-2000s that HIV-associated neurocognitive impairment was a global problem particularly in resource-limited countries (Wong et al. 2007; Nakasujja et al. 2005), we believe that it is critical to examine the neuroepidemiology of SARS-CoV-2 in resource-limited countries as soon as possible. Because of the respiratory mode of transmission of SARS-CoV-2 and limitations in personal protective equipment and medical supplies in hospitals in resource-limited countries, it is likely that infection rates will be in much higher numbers. In particular, Latin America, where standard public health policies to counter the pandemic are often being ignored by local government officials or even top leaders (e.g., Brazil), and sub-Saharan Africa, in which a majority of residents live in densely populated communities where social distancing is not possible, are two areas that are particularly susceptible to rampant SARS-CoV-2 infection. Preventing the spread of infection requires basic resources like those to permit frequent handwashing (i.e., clean water and soap) and social distancing (e.g., adequate housing). Establishment of international cohorts will also allow for the delineation of genetic, environmental, and cultural factors involved in neuropsychological outcomes of SARS-CoV-2 infection.
Domestically, cohorts must include a broad cross section of races, oversampling (proportionally) for African-Americans and Latinos, while maintaining an equal representation of men and women across all groups. In addition, the samples must also represent a range of socio-economic strata, so as not to conflate “race” with other social factors. Finally, appropriate at-risk individuals must be included—that is individuals who, based on occupation, lifestyle, or other factors, may be at greater risk for infection than the population as a whole.
Perhaps drawing from the experiences with HIV-1 and other pandemic viruses and recognizing that early action will beget stronger prevention and intervention, the National Institutes of Health and other public and private funding sources have already begun soliciting grant applications that leverage the infrastructure of extant cohort studies, including those of HIV-1, which will more quickly generate epidemiological data that can effectively shape public health policy and pharmaceutical development efforts. This approach will also generate important data concerning HIV-1/SARS-CoV-2 coinfection.
Conclusion
The SARS-CoV-2 pandemic is like nothing the world has seen in modern times. However, thanks to modern technology and scientific thinking, elucidating host susceptibility factors (e.g., genetic, medical, psychosocial) and tracking the long-term effects of the virus and its associated syndrome are already being implemented or planned. Thinking ahead, long-term monitoring of SARS-CoV-2 antibody–positive individuals and uninfected controls in cohort studies is required to determine if the COVID-19 and/or asymptomatic infection has later effects on neuropsychological functioning while also considering potential mediating or contributing factors, as has been done with HIV-1 (Sacktor et al. 2016; Rubin et al. 2017; Kaslow et al. 1987; Wang et al. 2019b; Barkan et al. 1998). Thus, drawing from lessons learned from over three decades of domestic neuroHIV research, as well as more recent international research, the scientific community will be better prepared to effectively design and execute such studies, as well as interpret their findings.
References
Adle-Biassette H, Chretien F, Wingertsmann L, Hery C, Ereau T, Scaravilli F, Tardieu M, Gray F (1999) Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol 25(2):123–133
Agarwal S, Jain R, Dogra S, Krieger P, Lewis A, Nguyen V, Melmed K, Galetta S (2020) Cerebral microbleeds and leukoencephalopathy in critically ill patients with COVID-19. Stroke. https://doi.org/10.1161/STROKEAHA.120.030940
Ahles TA, Root JC (2018) Cognitive effects of cancer and cancer treatments. Annu Rev Clin Psychol 14:425–451. https://doi.org/10.1146/annurev-clinpsy-050817-084903
Alvarez-Carbonell D, Ye F, Ramanath N, Garcia-Mesa Y, Knapp PE, Hauser KF, Karn J (2019) Cross-talk between microglia and neurons regulates HIV latency. PLoS Pathog 15(12):e1008249. https://doi.org/10.1371/journal.ppat.1008249
Ancuta P, Moses A, Gabuzda D (2004) Transendothelial migration of CD16+ monocytes in response to fractalkine under constitutive and inflammatory conditions. Immunobiology. 209(1-2):11–20
Andries K, Pensaert MB (1980) Immunofluorescence studies on the pathogenesis of hemagglutinating encephalomyelitis virus infection in pigs after oronasal inoculation. Am J Vet Res 41(9):1372–1378
Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 69(18):1789–1799
Arbour N, Ekande S, Cote G, Lachance C, Chagnon F, Tardieu M, Cashman NR, Talbot PJ (1999) Persistent infection of human oligodendrocytic and neuroglial cell lines by human coronavirus 229E. J Virol 73(4):3326–3337
Arbour N, Day R, Newcombe J, Talbot PJ (2000) Neuroinvasion by human respiratory coronaviruses. J Virol 74(19):8913–8921. https://doi.org/10.1128/jvi.74.19.8913-8921.2000
Argenziano B, Slater, Tiao, Baldwin, Barr, Chang, et al. (2020) Characterization and clinical course of 1000 patients with COVID-19 in New York: retrospective case series. BMJ. Available from: https://doi.org/10.1101/2020.04.20.20072116.
Argyris EG, Acheampong E, Wang F, Huang J, Chen K, Mukhtar M, Zhang H et al (2007) Virology 367(2):440–451. https://doi.org/10.1016/j.virol.2007.06.010
Arrildt KT, Joseph SB, Swanstrom R (2012) The HIV-1 env protein: a coat of many colors. Curr HIV/AIDS Rep 9(1):52–63. https://doi.org/10.1007/s11904-011-0107-3
Ashrafi F, Zali A, Ommi D, Salari M, Fatemi A, Arab-Ahmadi M, Behnam B, Azhideh A, Vahidi M, Yousefi-Asl M, Jalili Khoshnood R, Advani S (2020) COVID-19-related strokes in adults below 55 years of age: a case series. Neurol Sci. https://doi.org/10.1007/s10072-020-04521-3
Atluri VS, Hidalgo M, Samikkannu T, Kurapati KR, Jayant RD, Sagar V, Nair MP (2015) Effect of human immunodeficiency virus on blood-brain barrier integrity and function: an update. Front Cell Neurosci 9:212. https://doi.org/10.3389/fncel.2015.00212
Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J (1998) The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 9(2):117–125
Becker JT, Lopez OL, Dew MA, Aizenstein HJ (2004) Prevalence of cognitive disorders differs as a function of age in HIV virus infection. Aids. 18(Suppl 1):S11–S18
Becker JT, Kingsley LA, Molsberry S, Reynolds S, Aronow A, Levine AJ, Martin E, Miller EN, Munro CA, Ragin A, Sacktor N, Selnes OA (2014) Cohort profile: recruitment cohorts in the neuropsychological substudy of the Multicenter AIDS Cohort Study. Int J Epidemiol. https://doi.org/10.1093/ije/dyu092
Bell JE, Brettle RP, Chiswick A, Simmonds P (1998) HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 121(Pt 11):2043–2052
Bender SJ, Weiss SR (2010) Pathogenesis of murine coronavirus in the central nervous system. J NeuroImmune Pharmacol 5(3):336–354. https://doi.org/10.1007/s11481-010-9202-2
Bennett SJ, Sauve MJ (2003) Cognitive deficits in patients with heart failure: a review of the literature. J Cardiovasc Nurs 18(3):219–242. https://doi.org/10.1097/00005082-200307000-00007
van den Berg E, Reijmer YD, de Bresser J, Kessels RP, Kappelle LJ, Biessels GJ (2010) Utrecht Diabetic Encephalopathy Study G. A 4 year follow-up study of cognitive functioning in patients with type 2 diabetes mellitus. Diabetologia 53(1):58–65. https://doi.org/10.1007/s00125-009-1571-9
Binder LM, Iverson GL, Brooks BL (2009) To err is human: “abnormal” neuropsychological scores and variability are common in healthy adults. Arch Clin Neuropsychol 24(1):31–46. https://doi.org/10.1093/arclin/acn001
Bonnet F, Amieva H, Marquant F, Bernard C, Bruyand M, Dauchy FA, Mercie P, Greib C, Richert L, Neau D, Catheline G, Dehail P, Dabis F, Morlat P, Dartigues JF, Chene G, Cohort SCA (2013) Cognitive disorders in HIV-infected patients: are they HIV-related? AIDS. 27(3):391–400. https://doi.org/10.1097/QAD.0b013e32835b1019
Boven LA (2000) Macrophages and HIV-1-associated dementia. Arch Immunol Ther Exp (Warsz) 48(4):273–279
Boven LA, Middel J, Breij EC, Schotte D, Verhoef J, Soderland C, Nottet HS (2000) Interactions between HIV-infected monocyte-derived macrophages and human brain microvascular endothelial cells result in increased expression of CC chemokines. J Neuro-Oncol 6(5):382–389
Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, Chance R, Macaulay IC, Chou HJ, Fletcher RB, Das D, Street K, Roux de Bezieux H, Choi YG, Risso D, Dudoit S, Purdom E, Mill J, Abi Hachem R, Matsunami H, Logan DW, Goldstein BJ, Grubb MS, Ngai J, Datta SR (2020) Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. https://doi.org/10.1126/sciadv.abc5801
Burks JS, DeVald BL, Jankovsky LD, Gerdes JC (1980) Two coronaviruses isolated from central nervous system tissue of two multiple sclerosis patients. Science. 209(4459):933–934. https://doi.org/10.1126/science.7403860
Chan DC, Fass D, Berger JM, Kim PS (1997) Core structure of gp41 from the HIV envelope glycoprotein. Cell. 89(2):263–273. https://doi.org/10.1016/s0092-8674(00)80205-6
Channappanavar R, Zhao J, Perlman S (2014) T cell-mediated immune response to respiratory coronaviruses. Immunol Res 59(1-3):118–128. https://doi.org/10.1007/s12026-014-8534-z
Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J (1996) The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 85(7):1135–1148
Clark IA, Vissel B (2017) The meteorology of cytokine storms, and the clinical usefulness of this knowledge. Semin Immunopathol 39(5):505–516. https://doi.org/10.1007/s00281-017-0628-y
Coakley E, Petropoulos CJ, Whitcomb JM (2005) Assessing chemokine co-receptor usage in HIV. Curr Opin Infect Dis 18(1):9–15. https://doi.org/10.1097/00001432-200502000-00003
Collins AR (2002) In vitro detection of apoptosis in monocytes/macrophages infected with human coronavirus. Clin Diagn Lab Immunol 9(6):1392–1395. https://doi.org/10.1128/cdli.9.6.1392-1395.2002
Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO (1998) Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A 95(6):3117–3121
Condie LO (2020) Neurotropic mechanisms in COVID-19 and their potential influence on neuropsychological outcomes in children. Child Neuropsychol:1–20. https://doi.org/10.1080/09297049.2020.1763938
Crews L, Lentz MR, Gonzalez RG, Fox HS, Masliah E (2008) Neuronal injury in simian immunodeficiency virus and other animal models of neuroAIDS. J Neuro-Oncol 14(4):327–339. https://doi.org/10.1080/13550280802132840
Cristallo A, Gambaro F, Biamonti G, Ferrante P, Battaglia M, Cereda PM (1997) Human coronavirus polyadenylated RNA sequences in cerebrospinal fluid from multiple sclerosis patients. New Microbiol 20(2):105–114
Cunningham AL, Donaghy H, Harman AN, Kim M, Turville SG (2010) Manipulation of dendritic cell function by viruses. Curr Opin Microbiol 13(4):524–529. https://doi.org/10.1016/j.mib.2010.06.002
Cysique LA, Brew BJ (2019) Comorbid depression and apathy in HIV-associated neurocognitive disorders in the era of chronic HIV infection. Handb Clin Neurol 165:71–82. https://doi.org/10.1016/B978-0-444-64012-3.00006-X
Cysique LA, Maruff P, Brew BJ (2004) Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neuro-Oncol 10(6):350–357
Dahm T, Rudolph H, Schwerk C, Schroten H, Tenenbaum T (2016) Neuroinvasion and inflammation in viral central nervous system infections. Mediat Inflamm 2016:8562805. https://doi.org/10.1155/2016/8562805
Desforges M, Miletti TC, Gagnon M, Talbot PJ (2007) Activation of human monocytes after infection by human coronavirus 229E. Virus Res 130(1-2):228–240. https://doi.org/10.1016/j.virusres.2007.06.016
Desforges M, Le Coupanec A, Stodola JK, Meessen-Pinard M, Talbot PJ (2014a) Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res 194:145–158. https://doi.org/10.1016/j.virusres.2014.09.011
Desforges M, Le Coupanec A, Brison E, Meessen-Pinard M, Talbot PJ (2014b) Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol 807:75–96. https://doi.org/10.1007/978-81-322-1777-0_6
Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dube M, Talbot PJ (2019) Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 12(1). https://doi.org/10.3390/v12010014
Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, Margolick JB (2011) A frailty-related phenotype before HAART initiation as an independent risk factor for AIDS or death after HAART among HIV-infected men. J Gerontol Ser A Biol Med Sci 66A(9):1030–1038. https://doi.org/10.1093/gerona/glr097
Dijkman R, Jebbink MF, Koekkoek SM, Deijs M, Jonsdottir HR, Molenkamp R, Ieven M, Goossens H, Thiel V, van der Hoek L (2013) Isolation and characterization of current human coronavirus strains in primary human epithelial cell cultures reveal differences in target cell tropism. J Virol 87(11):6081–6090. https://doi.org/10.1128/JVI.03368-12
Dore GJ, McDonald A, Li Y, Kaldor JM, Brew BJ (2003) Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. Aids. 17(10):1539–1545
Dube M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ (2018) Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol 92(17). https://doi.org/10.1128/JVI.00404-18
Eliezer M, Hautefort C, Hamel AL, Verillaud B, Herman P, Houdart E, Eloit C (2020) Sudden and complete olfactory loss function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg. https://doi.org/10.1001/jamaoto.2020.0832
Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S, Crowe SM (2007) The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol 178(10):6581–6589
Erdmann N, Heath SL (2019) Cytokine storm syndrome as a manifestation of primary HIV infection. In: Cron RQ, Behrens EM (eds) Cytokine storm syndrome. Springer International Publishing, Cham, pp 299–306
Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW (2006) CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci 26(4):1098–1106. https://doi.org/10.1523/JNEUROSCI.3863-05.2006
Everall IP, Hansen LA, Masliah E (2005) The shifting patterns of HIV encephalitis neuropathology. Neurotox Res 8(1-2):51–61
Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, Moore D, Ellis R, Cherner M, Gelman B, Morgello S, Singer E, Grant I, Masliah E, National Neuro ATC (2009) Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neuro-Oncol 15(5-6):360–370. https://doi.org/10.3109/13550280903131915
Fazzini E, Fleming J, Fahn S (1992) Cerebrospinal fluid antibodies to coronavirus in patients with Parkinson’s disease. Mov Disord 7(2):153–158. https://doi.org/10.1002/mds.870070210
Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, Rusconi S, Gervasoni C, Ridolfo AL, Rizzardini G, Antinori S, Galli M. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis 2020. https://doi.org/10.1093/cid/ciaa330.
Gisslen M, Price RW, Nilsson S (2011) The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect Dis 11:356. https://doi.org/10.1186/1471-2334-11-356
Glass JD, Fedor H, Wesselingh SL, McArthur JC (1995) Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol 38(5):755–762. https://doi.org/10.1002/ana.410380510
Glass WG, Subbarao K, Murphy B, Murphy PM (2004) Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J Immunol 173(6):4030–4039. https://doi.org/10.4049/jimmunol.173.6.4030
Gowrisankar YV, Clark MA (2016) Angiotensin II regulation of angiotensin-converting enzymes in spontaneously hypertensive rat primary astrocyte cultures. J Neurochem 138(1):74–85. https://doi.org/10.1111/jnc.13641
Gross AM, Jaeger PA, Kreisberg JF, Licon K, Jepsen KL, Khosroheidari M, Morsey BM, Swindells S, Shen H, Ng CT, Flagg K, Chen D, Zhang K, Fox HS, Ideker T (2016) Methylome-wide analysis of chronic HIV infection reveals five-year increase in biological age and epigenetic targeting of HLA. Mol Cell 62(2):157–168. https://doi.org/10.1016/j.molcel.2016.03.019
Gu CTKZWWZPZLPYZGJ (2020) Clinical characteristics of 20,662 patients with COVID-19 in mainland China: a systemic review and meta-analysis. Available from: https://www.medrxiv.org/content/10.1101/2020.04.18.20070565v1.
Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Zhan J, Wang S, Xie Z, Zhuang H, Wu B, Zhong H, Shao H, Fang W, Gao D, Pei F, Li X, He Z, Xu D, Shi X, Anderson VM, Leong AS (2005) Multiple organ infection and the pathogenesis of SARS. J Exp Med 202(3):415–424. https://doi.org/10.1084/jem.20050828
Guha D, Wagner MCE, Ayyavoo V (2018) Human immunodeficiency virus type 1 (HIV-1)-mediated neuroinflammation dysregulates neurogranin and induces synaptodendritic injury. J Neuroinflammation 15(1):126. https://doi.org/10.1186/s12974-018-1160-2
Gupta RK, Peppa D, Hill AL, Galvez C, Salgado M, Pace M, McCoy LE, Griffith SA, Thornhill J, Alrubayyi A, Huyveneers LEP, Nastouli E, Grant P, Edwards SG, Innes AJ, Frater J, Nijhuis M, Wensing AMJ, Martinez-Picado J, Olavarria E (2020) Evidence for HIV-1 cure after CCR5Delta32/Delta32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report. Lancet HIV. https://doi.org/10.1016/S2352-3018(20)30069-2
Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, JC MA, Morgello S, Simpson DM, JA MC, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C (2010) HIV-Associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75(23):2087–2096. https://doi.org/10.1212/WNL.0b013e318200d727
Heaton RK, Franklin DR, Ellis RJ, JA MC, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, JC MA, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neuro-Oncol 17(1):3–16
Heaton RK, Franklin DR Jr, Deutsch R, Letendre S, Ellis RJ, Casaletto K, Marquine MJ, Woods SP, Vaida F, Atkinson JH, Marcotte TD, JA MC, Collier AC, Marra CM, Clifford DB, Gelman BB, Sacktor N, Morgello S, Simpson DM, Abramson I, Gamst AC, Fennema-Notestine C, Smith DM, Grant I, Group C (2015) Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis 60(3):473–480. https://doi.org/10.1093/cid/ciu862
Heaton RK, Franklin DR, Ellis RJ, JA MC, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, JC MA, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, for the C, Groups H HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neuro-Oncol 17:3. https://doi.org/10.1007/s13365-010-0006-1
Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, Anheim M, Meziani F (2020) Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 382:2268. https://doi.org/10.1056/NEJMc2008597
Hoesch RE, Lin E, Young M, Gottesman RF, Altaweel L, Nyquist PA, Stevens RD (2012) Acute lung injury in critical neurological illness. Crit Care Med 40(2):587–593. https://doi.org/10.1097/CCM.0b013e3182329617
Hong X, Currier GW, Zhao X, Jiang Y, Zhou W, Wei J (2009) Posttraumatic stress disorder in convalescent severe acute respiratory syndrome patients: a 4-year follow-up study. Gen Hosp Psychiatry 31(6):546–554. https://doi.org/10.1016/j.genhosppsych.2009.06.008
Hopkins RO, Gale SD, Weaver LK (2006) Brain atrophy and cognitive impairment in survivors of acute respiratory distress syndrome. Brain Inj 20(3):263–271. https://doi.org/10.1080/02699050500488199
Horvath S, Levine AJ (2015) HIV-1 infection accelerates age according to the epigenetic clock. J Infect Dis. https://doi.org/10.1093/infdis/jiv277
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O, Blau IW, Hofmann WK, Thiel E (2009) Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 360(7):692–698. https://doi.org/10.1056/NEJMoa0802905
Imamura S, Ishihara Y, Hattori T, Kurasawa O, Matsushita Y, Sugihara Y, Kanzaki N, Iizawa Y, Baba M, Hashiguchi S (2004) CCR5 antagonists as anti-HIV-1 agents. 1. Synthesis and biological evaluation of 5-oxopyrrolidine-3-carboxamide derivatives. Chem Pharm Bull (Tokyo) 52(1):63–73
Itzhaki RF, Dobson CB, Wozniak MA (2004) Herpes simplex virus type 1 and Alzheimer’s disease. Ann Neurol 55(2):299–300; author reply -1. https://doi.org/10.1002/ana.10852
Janelsins MC, Heckler CE, Peppone LJ, Ahles TA, Mohile SG, Mustian KM, Palesh O, O'Mara AM, Minasian LM, Williams AM, Magnuson A, Geer J, Dakhil SR, Hopkins JO, Morrow GR (2018) Longitudinal trajectory and characterization of cancer-related cognitive impairment in a nationwide cohort study. J Clin Oncol:JCO2018786624. https://doi.org/10.1200/JCO.2018.78.6624
Jang H, Boltz D, Sturm-Ramirez K, Shepherd KR, Jiao Y, Webster R, Smeyne RJ (2009) Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci U S A 106(33):14063–14068. https://doi.org/10.1073/pnas.0900096106
Ji W, Wang W, Zhao X, Zai J, Li X (2020) Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol 92(4):433–440. https://doi.org/10.1002/jmv.25682
Johnson-Lussenburg CM, Zheng Q (1987) Coronavirus and multiple sclerosis: results of a case/control longitudinal serological study. Adv Exp Med Biol 218:421–429. https://doi.org/10.1007/978-1-4684-1280-2_51
Kallianpur AR, Levine AJ (2014) Host genetic factors predisposing to HIV-associated neurocognitive disorder. Curr HIV/AIDS Rep. https://doi.org/10.1007/s11904-014-0222-z
Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr (1987) The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 126(2):310–318
Kaul M, Lipton SA (2006) Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res 4(3):307–318
Kedzierska K, Crowe SM (2002) The role of monocytes and macrophages in the pathogenesis of HIV-1 infection. Curr Med Chem 9(21):1893–1903
Kim WK, Corey S, Alvarez X, Williams K (2003) Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol 74(5):650–656. https://doi.org/10.1189/jlb.0503207
Kim EY, Liao Q, Yu ES, Kim JH, Yoon SW, Lam WW, Fielding R (2016) Middle East respiratory syndrome in South Korea during 2015: risk-related perceptions and quarantine attitudes. Am J Infect Control 44(11):1414–1416. https://doi.org/10.1016/j.ajic.2016.03.014
Kim JE, Heo JH, Kim HO, Song SH, Park SS, Park TH, Ahn JY, Kim MK, Choi JP (2017) Neurological complications during treatment of Middle East respiratory syndrome. J Clin Neurol 13(3):227–233. https://doi.org/10.3988/jcn.2017.13.3.227
Kirk GD, Mehta SH, Astemborski J, Galai N, Washington J, Higgins Y, Balagopal A, Thomas DL (2013;N/A(N/A):N/A-N/A) HIV, age, and the severity of hepatitis C virus–related liver disease: a cohort study. Ann Intern Med. https://doi.org/10.7326/0003-4819-158-9-201305070-00604
Kraft-Terry SD, Buch SJ, Fox HS, Gendelman HE (2009) A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron 64(1):133–145. https://doi.org/10.1016/j.neuron.2009.09.042
Kurella Tamura M, Xie D, Yaffe K, Cohen DL, Teal V, Kasner SE, Messe SR, Sehgal AR, Kusek J, DeSalvo KB, Cornish-Zirker D, Cohan J, Seliger SL, Chertow GM, Go AS et al (2011) Clin J Am Soc Nephrol 6(2):248–256. https://doi.org/10.2215/CJN.02660310
Lake JE, Popov M, Post WS, Palella FJ, Sacktor N, Miller EN, Brown TT, Becker JT (2017) Visceral fat is associated with brain structure independent of human immunodeficiency virus infection status. J Neuro-Oncol 23(3):385–393. https://doi.org/10.1007/s13365-016-0507-7
Lam TT, Shum MH, Zhu HC, Tong YG, Ni XB, Liao YS, Wei W, Cheung WY, Li WJ, Li LF, Leung GM, Holmes EC, Hu YL, Guan Y (2020) Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature 538:282. https://doi.org/10.1038/s41586-020-2169-0
Leibovitch EC, Jacobson S (2018) Viruses in chronic progressive neurologic disease. Mult Scler 24(1):48–52. https://doi.org/10.1177/1352458517737392
Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ (2008) Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 65(1):65–70
Letendre SL, Zheng JC, Kaul M, Yiannoutsos CT, Ellis RJ, Taylor MJ, Marquie-Beck J, Navia B (2011) Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J Neuro-Oncol 17(1):63–69. https://doi.org/10.1007/s13365-010-0013-2
Levine AJ, Hinkin CH, Miller EN, Becker JT, Selnes OA, Cohen BA (2007) The generalizability of neurocognitive test/retest data derived from a nonclinical sample for detecting change among two HIV+ cohorts. J Clin Exp Neuropsychol 29(6):669–678
Levine AJ, Horvath S, Miller EN, Singer EJ, Shapshak P, Baldwin GC, Martinez-Maza O, Witt MD, Langfelder P (2013) Transcriptome analysis of HIV-infected peripheral blood monocytes: gene transcripts and networks associated with neurocognitive functioning. J Neuroimmunol 265(1-2):96–105. https://doi.org/10.1016/j.jneuroim.2013.09.016
Levine AJ, Soontornniyomkij V, Achim CL, Masliah E, Gelman BB, Sinsheimer JS, Singer EJ, Moore DJ (2015) Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J Neuro-Oncol. https://doi.org/10.1007/s13365-015-0410-7
Li YC, Bai WZ, Hirano N, Hayashida T, Hashikawa T (2012) Coronavirus infection of rat dorsal root ganglia: ultrastructural characterization of viral replication, transfer, and the early response of satellite cells. Virus Res 163(2):628–635. https://doi.org/10.1016/j.virusres.2011.12.021
Li YC, Bai WZ, Hirano N, Hayashida T, Taniguchi T, Sugita Y, Tohyama K, Hashikawa T (2013) Neurotropic virus tracing suggests a membranous-coating-mediated mechanism for transsynaptic communication. J Comp Neurol 521(1):203–212. https://doi.org/10.1002/cne.23171
Li K, Wohlford-Lenane C, Perlman S, Zhao J, Jewell AK, Reznikov LR, Gibson-Corley KN, Meyerholz DK, McCray PB Jr (2016a) Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis 213(5):712–722. https://doi.org/10.1093/infdis/jiv499
Li Y, Li H, Fan R, Wen B, Zhang J, Cao X, Wang C, Song Z, Li S, Li X, Lv X, Qu X, Huang R, Liu W (2016b) Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 59(3):163–169. https://doi.org/10.1159/000453066
Li YC, Bai WZ, Hashikawa T (2020) The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. https://doi.org/10.1002/jmv.25728
Lindl KA, Akay C, Wang Y, White MG, Jordan-Sciutto KL (2007) Expression of the endoplasmic reticulum stress response marker, BiP, in the central nervous system of HIV-positive individuals. Neuropathol Appl Neurobiol 33(6):658–669. https://doi.org/10.1111/j.1365-2990.2007.00866.x
Liu N, Zhang F, Wei C, Jia Y, Shang Z, Sun L, Wu L, Sun Z, Zhou Y, Wang Y, Liu W (2020) Prevalence and predictors of PTSS during COVID-19 outbreak in China hardest-hit areas: gender differences matter. Psychiatry Res 287:112921. https://doi.org/10.1016/j.psychres.2020.112921
Lochhead JJ, Thorne RG (2012) Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev 64(7):614–628. https://doi.org/10.1016/j.addr.2011.11.002
Lochhead JJ, Kellohen KL, Ronaldson PT, Davis TP (2019) Distribution of insulin in trigeminal nerve and brain after intranasal administration. Sci Rep 9(1):2621. https://doi.org/10.1038/s41598-019-39191-5
Lu H, Surkan PJ, Irwin MR, Treisman GJ, Breen EC, Sacktor N, Stall R, Wolinsky SM, Jacobson LP, Abraham AG (2019) Inflammation and risk of depression in HIV: prospective findings from the Multicenter AIDS Cohort Study. Am J Epidemiol 188(11):1994–2003. https://doi.org/10.1093/aje/kwz190
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 395(10224):565–574. https://doi.org/10.1016/S0140-6736(20)30251-8
Lucas GM, Mehta SH, Atta MG, Kirk GD, Galai N, Vlahov D, Moore RD (2007) End-stage renal disease and chronic kidney disease in a cohort of African-American HIV-infected and at-risk HIV-seronegative participants followed between 1988 and 2004. AIDS. 21(18):2435–2443. https://doi.org/10.1097/QAD.0b013e32827038ad
Ludlow M, Kortekaas J, Herden C, Hoffmann B, Tappe D, Trebst C, Griffin DE, Brindle HE, Solomon T, Brown AS, van Riel D, Wolthers KC, Pajkrt D, Wohlsein P, Martina BEE, Baumgartner W, Verjans GM, Osterhaus A (2016) Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol 131(2):159–184. https://doi.org/10.1007/s00401-015-1511-3
Majde JA (2010) Neuroinflammation resulting from covert brain invasion by common viruses - a potential role in local and global neurodegeneration. Med Hypotheses 75(2):204–213. https://doi.org/10.1016/j.mehy.2010.02.023
Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, Perschler P, Gould F, Martin E (2009) Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology 72(19):1661–1668. https://doi.org/10.1212/WNL.0b013e3181a55f65
Manzardo C, Guardo AC, Letang E, Plana M, Gatell JM, Miro JM (2015) Opportunistic infections and immune reconstitution inflammatory syndrome in HIV-1-infected adults in the combined antiretroviral therapy era: a comprehensive review. Expert Rev Anti-Infect Ther 13(6):751–767. https://doi.org/10.1586/14787210.2015.1029917
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77:1. https://doi.org/10.1001/jamaneurol.2020.1127
Matsuda K, Park CH, Sunden Y, Kimura T, Ochiai K, Kida H, Umemura T (2004) The vagus nerve is one route of transneural invasion for intranasally inoculated influenza a virus in mice. Vet Pathol 41(2):101–107. https://doi.org/10.1354/vp.41-2-101
Maunder RG, Lancee WJ, Rourke S, Hunter JJ, Goldbloom D, Balderson K, Petryshen P, Steinberg R, Wasylenki D, Koh D, Fones CS (2004) Factors associated with the psychological impact of severe acute respiratory syndrome on nurses and other hospital workers in Toronto. Psychosom Med 66(6):938–942. https://doi.org/10.1097/01.psy.0000145673.84698.18
McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP et al (1993) Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology 43(11):2245–2252
McArthur JC, Brew BJ, Nath A (2005) Neurological complications of HIV infection. Lancet Neurol 4(9):543–555. https://doi.org/10.1016/S1474-4422(05)70165-4
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (2020) Hlh Across Speciality Collaboration UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 395(10229):1033–1034. https://doi.org/10.1016/S0140-6736(20)30628-0
Memish ZA, Mishra N, Olival KJ, Fagbo SF, Kapoor V, Epstein JH, Alhakeem R, Durosinloun A, Al Asmari M, Islam A, Kapoor A, Briese T, Daszak P, Al Rabeeah AA, Lipkin WI (2013) Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis 19(11):1819–1823. https://doi.org/10.3201/eid1911.131172
Meyer AC, Boscardin WJ, Kwasa JK, Price RW (2013) Is it time to rethink how neuropsychological tests are used to diagnose mild forms of HIV-associated neurocognitive disorders? Impact of false-positive rates on prevalence and power. Neuroepidemiology. 41(3-4):208–216. https://doi.org/10.1159/000354629
Meyer VJ, Little DM, Fitzgerald DA, Sundermann EE, Rubin LH, Martin EM, Weber KM, Cohen MH, Maki PM (2014) Crack cocaine use impairs anterior cingulate and prefrontal cortex function in women with HIV infection. J Neuro-Oncol 20(4):352–361. https://doi.org/10.1007/s13365-014-0250-x
Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, Ellis RJ, Achim CL, Marcotte TD, Heaton RK, Grant I (2006a) Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 20(6):879–887. https://doi.org/10.1097/01.aids.0000218552.69834.00
Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, Ellis RJ, Achim CL, Marcotte RD, Heaton RK, Grant I (2006b) Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. Aids. 20(6):879–887
Morfopoulou S, Brown JR, Davies EG, Anderson G, Virasami A, Qasim W, Chong WK, Hubank M, Plagnol V, Desforges M, Jacques TS, Talbot PJ, Breuer J (2016) Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med 375(5):497–498. https://doi.org/10.1056/NEJMc1509458
Mori I (2015) Transolfactory neuroinvasion by viruses threatens the human brain. Acta Virol 59(4):338–349. https://doi.org/10.4149/av_2015_04_338
Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S (2020) A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. https://doi.org/10.1016/j.ijid.2020.03.062
Murray RS, Brown B, Brian D, Cabirac GF (1992) Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann Neurol 31(5):525–533. https://doi.org/10.1002/ana.410310511
Nakasujja N, Musisi S, Robertson K, Wong M, Sacktor N, Ronald A (2005) Human immunodeficiency virus neurological complications: an overview of the Ugandan experience. J Neuro-Oncol 11(Suppl 3):26–29. https://doi.org/10.1080/13550280500511782
Nath A (2020) Neurologic complications of coronavirus infections. Neurology. https://doi.org/10.1212/WNL.0000000000009455
Navia BA, Jordan BD, Price RW (1986) The AIDS dementia complex: I. Clinical features. Ann Neurol 19(6):517–524
Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S (2008) Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 82(15):7264–7275. https://doi.org/10.1128/JVI.00737-08
Novak V, Hajjar I (2010) The relationship between blood pressure and cognitive function. Nat Rev Cardiol 7(12):686–698. https://doi.org/10.1038/nrcardio.2010.161
Nowacek AS, McMillan J, Miller R, Anderson A, Rabinow B, Gendelman HE (2010) Nanoformulated antiretroviral drug combinations extend drug release and antiretroviral responses in HIV-1-infected macrophages: implications for neuroAIDS therapeutics. J NeuroImmune Pharmacol 5(4):592–601. https://doi.org/10.1007/s11481-010-9198-7
Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, Skliut M, Weinberger J, Dangayach NS, Bederson JB, Tuhrim S, Fifi JT (2020) Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. https://doi.org/10.1056/NEJMc2009787
Ozkale Y, Erol I, Ozkale M, Demir S, Alehan F (2012) Acute disseminated encephalomyelitis associated with influenza A H1N1 infection. Pediatr Neurol 47(1):62–64. https://doi.org/10.1016/j.pediatrneurol.2012.03.019
Palmer BW, Boone KB, Lesser IM, Wohl MA (1998) Base rates of “impaired” neuropsychological test performance among healthy older adults. Arch Clin Neuropsychol 13(6):503–511
Park CH, Ishinaka M, Takada A, Kida H, Kimura T, Ochiai K, Umemura T (2002) The invasion routes of neurovirulent A/Hong Kong/483/97 (H5N1) influenza virus into the central nervous system after respiratory infection in mice. Arch Virol 147(7):1425–1436. https://doi.org/10.1007/s00705-001-0750-x
Patel AV, Wade JB, Thacker LR, Sterling RK, Siddiqui MS, Stravitz RT, Sanyal AJ, Luketic V, Puri P, Fuchs M, Matherly S, White MB, Unser A, Heuman DM, Bajaj JS (2015) Cognitive reserve is a determinant of health-related quality of life in patients with cirrhosis, independent of covert hepatic encephalopathy and model for end-stage liver disease score. Clin Gastroenterol Hepatol 13(5):987–991. https://doi.org/10.1016/j.cgh.2014.09.049
Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, Jayaseelan DL, Kumar G, Raftopoulos RE, Zambreanu L, Vivekanandam V, Khoo A, Geraldes R, Chinthapalli K, Boyd E, Tuzlali H, Price G, Christofi G, Morrow J, McNamara P, McLoughlin B, Lim ST, Mehta PR, Levee V, Keddie S, Yong W, Trip SA, AJM F, Hotton G, Miller TD, Everitt AD, Carswell C, NWS D, Yoong M, Attwell D, Sreedharan J, Silber E, Schott JM, Chandratheva A, Perry RJ, Simister R, Checkley A, Longley N, Farmer SF, Carletti F, Houlihan C, Thom M, Lunn MP, Spillane J, Howard R, Vincent A, Werring DJ, Hoskote C, Jager HR, Manji H, Zandi MS, Neurology UCLQSNHf, Neurosurgery C-SG (2020) The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. https://doi.org/10.1093/brain/awaa240
Peluso R, Haase A, Stowring L, Edwards M, Ventura P (1985) A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology. 147(1):231–236
Persidsky Y, Gendelman HE (2003) Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J Leukoc Biol 74(5):691–701
Pleasure SJ, Green AJ, Josephson SA (2020) The spectrum of neurologic disease in the severe acute respiratory syndrome coronavirus 2 pandemic infection: neurologists move to the frontlines. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.1065
Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B (2020) COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 201187. https://doi.org/10.1148/radiol.2020201187
Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS (1997) Unique monocyte subset in patients with AIDS dementia. Lancet. 349(9053):692–695. https://doi.org/10.1016/S0140-6736(96)10178-1
Reijmer YD, van den Berg E, de Bresser J, Kessels RP, Kappelle LJ, Algra A, Biessels GJ (2011) Utrecht Diabetic Encephalopathy Study G. Accelerated cognitive decline in patients with type 2 diabetes: MRI correlates and risk factors. Diabetes Metab Res Rev 27(2):195–202. https://doi.org/10.1002/dmrr.1163
Rickabaugh TM, Baxter RM, Sehl M, Sinsheimer JS, Hultin PM, Hultin LE, Quach A, Martinez-Maza O, Horvath S, Vilain E, Jamieson BD (2015) Acceleration of age-associated methylation patterns in HIV-1-infected adults. PLoS One 10(3):e0119201. https://doi.org/10.1371/journal.pone.0119201
Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ (2007) The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 21(14):1915–1921. https://doi.org/10.1097/QAD.0b013e32828e4e27
Rose NR (2017) Negative selection, epitope mimicry and autoimmunity. Curr Opin Immunol 49:51–55. https://doi.org/10.1016/j.coi.2017.08.014
Rubin LH, Maki PM (2019) HIV, depression, and cognitive impairment in the era of effective antiretroviral therapy. Curr HIV/AIDS Rep 16(1):82–95. https://doi.org/10.1007/s11904-019-00421-0
Rubin LH, Maki PM, Springer G, Benning L, Anastos K, Gustafson D, Villacres MC, Jiang X, Adimora AA, Waldrop-Valverde D, Vance DE, Bolivar H, Alden C, Martin EM, Valcour VG, Women's Interagency HIVS (2017) Cognitive trajectories over 4 years among HIV-infected women with optimal viral suppression. Neurology 89(15):1594–1603. https://doi.org/10.1212/WNL.0000000000004491
Rubin LH, Springer G, Martin EM, Seaberg EC, Sacktor NC, Levine A, Valcour VG, Young MA, Becker JT, Maki PM, Neuropsychology Working Groups of the Women's InterAgency HIVS, the Multicenter ACS (2019) Elevated depressive symptoms are a stronger predictor of executive dysfunction in HIV-infected women than men. J Acquir Immune Defic Syndr. https://doi.org/10.1097/QAI.0000000000002029
Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, Selim MA, Al Mutairi M, Al Nakhli D, Al Aidaroos AY, Al Sherbeeni N, Al-Khashan HI, Memish ZA, Albarrak AM (2014) Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis 29:301–306. https://doi.org/10.1016/j.ijid.2014.09.003
Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L (2002) HIV-associated cognitive impairment before and after the advent of combination therapy. J Neuro-Oncol 8(2):136–142
Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, Ragin A, Levine A, Miller E (2016) Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 86(4):334–340. https://doi.org/10.1212/WNL.0000000000002277
Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC (2016) HIV-associated neurocognitive disorder - pathogenesis and prospects for treatment. Nat Rev Neurol 12(5):309. https://doi.org/10.1038/nrneurol.2016.53
Schou L, Ostergaard B, Rasmussen LS, Rydahl-Hansen S, Phanareth K (2012) Cognitive dysfunction in patients with chronic obstructive pulmonary disease--a systematic review. Respir Med 106(8):1071–1081. https://doi.org/10.1016/j.rmed.2012.03.013
Schretlen DJ, Munro CA, Anthony JC, Pearlson GD (2003) Examining the range of normal intraindividual variability in neuropsychological test performance. J Int Neuropsychol Soc 9(6):864–870. https://doi.org/10.1017/S1355617703960061
Schretlen DJ, Testa SM, Winicki JM, Pearlson GD, Gordon B (2008) Frequency and bases of abnormal performance by healthy adults on neuropsychological testing. J Int Neuropsychol Soc 14(3):436–445. https://doi.org/10.1017/S1355617708080387
Sepehrinezhad A, Shahbazi A, Negah SS (2020) COVID-19 virus may have neuroinvasive potential and cause neurological complications: a perspective review. J Neuro-Oncol 26(3):324–329. https://doi.org/10.1007/s13365-020-00851-2
Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F (2020) Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A 117(21):11727–11734. https://doi.org/10.1073/pnas.2003138117
Siliciano RF, Greene WC (2011) HIV latency. Cold Spring Harb Perspect Med 1(1):a007096. https://doi.org/10.1101/cshperspect.a007096
Silverberg M, Chao C, Leyden W, Xu L, Tang B, Horberg M, Klein D, Quesenberry C, Towner W, Abrams D (2009) HIV infection and the risk of cancers with and without a known infectious cause. AIDS 23(17):2337–2345. https://doi.org/10.1097/QAD.0b013e3283319184
Silverberg MJ, Chao C, Leyden WA, Xu L, Horberg MA, Klein D, Towner WJ, Dubrow R, Quesenberry CP, Neugebauer RS, Abrams DI (2011) HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomark Prev 20(12):2551–2559. https://doi.org/10.1158/1055-9965.epi-11-0777
Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, Du Pasquier RA (2010) Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 24(9):1243–1250. https://doi.org/10.1097/QAD.0b013e3283354a7b
Smith AP (2012) Effects of the common cold on mood, psychomotor performance, the encoding of new information, speed of working memory and semantic processing. Brain Behav Immun 26(7):1072–1076. https://doi.org/10.1016/j.bbi.2012.06.012
Smith AP (2013) Twenty-five years of research on the behavioural malaise associated with influenza and the common cold. Psychoneuroendocrinology 38(6):744–751. https://doi.org/10.1016/j.psyneuen.2012.09.002
Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, Adams G, Hornick JL, Padera RF Jr, Sabeti P (2020) Neuropathological features of COVID-19. N Engl J Med. https://doi.org/10.1056/NEJMc2019373
South AM, Diz D, Chappell MC (2020) COVID-19, ACE2 and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. https://doi.org/10.1152/ajpheart.00217.2020
Spies G, Konkiewitz EC, Seedat S (2018) Incidence and persistence of depression among women living with and without HIV in South Africa: a longitudinal study. AIDS Behav 22(10):3155–3165. https://doi.org/10.1007/s10461-018-2072-y
Stawiski E DD, Suryamohan K, Gupta R, Fellouse FA, Sathirapongsasuti JF, et al. (2020) Human ACE2 receptor polymorphisms predict SARS-CoV-2 susceptibility. Available from: https://www.biorxiv.org/content/10.1101/2020.04.07.024752v1.
Stewart JN, Mounir S, Talbot PJ (1992) Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. 191(1):502–505. https://doi.org/10.1016/0042-6822(92)90220-j
Su T, Schouten J, Geurtsen GJ, Wit FW, Stolte IG, Prins M, Portegies P, Caan MW, Reiss P, Majoie CB, Schmand BA, Group AGCS (2015) Multivariate normative comparison, a novel method for more reliably detecting cognitive impairment in HIV infection. AIDS. 29(5):547–557. https://doi.org/10.1097/QAD.0000000000000573
Sun LSZ, Wu L, Zhu Z, Zhang F, Shang Z, Jia Y, Gu J, Zhou Y, Wang Y, Liu N, Liu W (2020) Prevalence and risk factors of acute posttraumatic stress symptoms during the COVID-19 outbreak in Wuhan, China. Available from: https://doi.org/10.1101/2020.03.06.20032425.
Talbot PJ, Ekande S, Cashman NR, Mounir S, Stewart JN (1993) Neurotropism of human coronavirus 229E. Adv Exp Med Biol 342:339–346. https://doi.org/10.1007/978-1-4615-2996-5_52
Tam CW, Pang EP, Lam LC, Chiu HF (2004) Severe acute respiratory syndrome (SARS) in Hong Kong in 2003: stress and psychological impact among frontline healthcare workers. Psychol Med 34(7):1197–1204. https://doi.org/10.1017/s0033291704002247
Toovey S (2008) Influenza-associated central nervous system dysfunction: a literature review. Travel Med Infect Dis 6(3):114–124. https://doi.org/10.1016/j.tmaid.2008.03.003
Troyer EA, Kohn JN, Hong S (2020) Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. https://doi.org/10.1016/j.bbi.2020.04.027
Tsai LK, Hsieh ST, Chang YC (2005) Neurological manifestations in severe acute respiratory syndrome. Acta Neurol Taiwanica 14(3):113–119
Vance DE, Rubin LH, Valcour V, Waldrop-Valverde D, Maki PM (2016) Aging and neurocognitive functioning in HIV-infected women: a review of the literature involving the women's interagency HIV study. Curr HIV/AIDS Rep 13(6):399–411. https://doi.org/10.1007/s11904-016-0340-x
Wallet C, De Rovere M, Van Assche J, Daouad F, De Wit S, Gautier V, Mallon PWG, Marcello A, Van Lint C, Rohr O, Schwartz C (2019) Microglial cells: the main HIV-1 reservoir in the brain. Front Cell Infect Microbiol 9(362). https://doi.org/10.3389/fcimb.2019.00362
Wang H, Sun J, Goldstein H (2008) Human immunodeficiency virus type 1 infection increases the in vivo capacity of peripheral monocytes to cross the blood-brain barrier into the brain and the in vivo sensitivity of the blood-brain barrier to disruption by lipopolysaccharide. J Virol 82(15):7591–7600. https://doi.org/10.1128/JVI.00768-08
Wang GF, Li W, Li K (2010) Acute encephalopathy and encephalitis caused by influenza virus infection. Curr Opin Neurol 23(3):305–311. https://doi.org/10.1097/wco.0b013e328338f6c9
Wang Z, Molsberry SA, Cheng Y, Kingsley L, Levine AJ, Martin E, Munro CA, Ragin A, Rubin LH, Sacktor N, Seaberg EC, Becker JT (2019a) Neuropsychology Working Group of the Multicenter ACS. Cross-sectional analysis of cognitive function using multivariate normative comparisons in men with HIV disease. AIDS. 33(14):2115–2124. https://doi.org/10.1097/QAD.0000000000002312
Wang Z, Molsberry SA, Cheng Y, Kingsley L, Levine AJ, Martin E, Munro CA, Ragin A, Rubin LH, Sacktor N, Seaberg EC, Becker JT, Neuropsychology Working Group of the Multicenter ACS (2019b) Cross-sectional analysis of cognitive function in the MACS with multivariate normative comparisons. AIDS. https://doi.org/10.1097/QAD.0000000000002312
Wang Y, Wang Y, Chen Y, Qin Q (2020) Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. https://doi.org/10.1002/jmv.25748
Womack JA, Goulet JL, Gibert C, Brandt C, Chang CC, Gulanski B, Fraenkel L, Mattocks K, Rimland D, Rodriguez-Barradas MC, Tate J, Yin MT, Justice AC, for the Veterans Aging Cohort Study Project T (2011) Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS One 6(2):e17217. https://doi.org/10.1371/journal.pone.0017217
Wong MH, Robertson K, Nakasujja N, Skolasky R, Musisi S, Katabira E, McArthur JC, Ronald A, Sacktor N (2007) Frequency of and risk factors for HIV dementia in an HIV clinic in sub-Saharan Africa. Neurology. 68(5):350–355
Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Hinkin CH, Lazzaretto D, Cherner M, Marcotte TD, Gelman BB, Morgello S, Singer EJ, Grant I, Heaton RK (2004) Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol 26(6):759–778
World Health Organization: Coronavirus disease (COVID-19) Pandemic (n.d.) Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
World Health Organization: Naming the coronavirus disease (COVID-19) and the virus that causes it (2020). Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it.
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 367(6483):1260–1263. https://doi.org/10.1126/science.abb2507
Wu M, Fatukasi O, Yang S, Alger J, Barker PB, Hetherington H, Kim T, Levine A, Martin E, Munro CA, Parrish T, Ragin A, Sacktor N, Seaberg E, Becker JT (2018) Neuropsychology Working Group of the Multicenter ACS. HIV disease and diabetes interact to affect brain white matter hyperintensities and cognition. AIDS 32(13):1803–1810. https://doi.org/10.1097/QAD.0000000000001891
Xia H, Lazartigues E (2010) Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep 12(3):170–175. https://doi.org/10.1007/s11906-010-0105-7
Xu J, Zhong S, Liu J, Li L, Li Y, Wu X, Li Z, Deng P, Zhang J, Zhong N, Ding Y, Jiang Y (2005) Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis 41(8):1089–1096. https://doi.org/10.1086/444461
Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H (2004) Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 113(1 Pt 1):e73–e76. https://doi.org/10.1542/peds.113.1.e73
Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y, Zhang S (2020) Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. https://doi.org/10.1056/NEJMc2007575
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579(7798):270–273. https://doi.org/10.1038/s41586-020-2012-7
Funding
Dr. Levine is supported by the National Institute of Neurological Disorders and Stroke grant R03-NS110476 and National Institute of Mental Health grant R21-MH115825. Dr. Sacktor is supported by the National Institute of Mental Health grants P30-MH075673 and R01-MH120693. Dr. Becker is supported by the National Institute on Aging grants R01-AG034852 and UF1-AG051197.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Levine, A., Sacktor, N. & Becker, J.T. Studying the neuropsychological sequelae of SARS-CoV-2: lessons learned from 35 years of neuroHIV research. J. Neurovirol. 26, 809–823 (2020). https://doi.org/10.1007/s13365-020-00897-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-020-00897-2