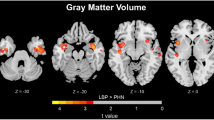
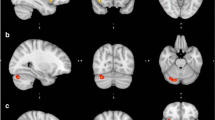
Abstract
We previously reported that neuropathic pain was associated with smaller posterior cingulate cortical (PCC) volumes, suggesting that a smaller/dysfunctional PCC may contribute to development of pain via impaired mind wandering. A gap in our previous report was lack of evidence for a mechanism for the genesis of PCC atrophy in HIV peripheral neuropathy. Here we investigate if volumetric differences in the subcortex for those with neuropathic paresthesia may contribute to smaller PCC volumes, potentially through deafferentation of ascending white matter tracts resulting from peripheral nerve damage in HIV neuropathy. Since neuropathic pain and paresthesia are highly correlated, statistical decomposition was used to separate pain and paresthesia symptoms to determine which regions of brain atrophy are associated with both pain and paresthesia and which are associated separately with pain or paresthesia. HIV+ individuals (N = 233) with and without paresthesia in a multisite study underwent structural brain magnetic resonance imaging. Voxel-based morphometry and a segmentation/registration tool were used to investigate regional brain volume changes associated with paresthesia. Analysis of decomposed variables found that smaller midbrain and thalamus volumes were associated with paresthesia rather than pain. However, atrophy in the PCC was related to both pain and paresthesia. Peak thalamic atrophy (p = 0.004; MNI x = − 14, y = − 24, z = − 2) for more severe paresthesia was in a region with reciprocal connections with the PCC. This provides initial evidence that smaller PCC volumes in HIV peripheral neuropathy are related to ascending white matter deafferentation caused by small fiber damage observed in HIV peripheral neuropathy.



Similar content being viewed by others
References
Albin KC, Simons CT (2010) Psychophysical evaluation of a sanshool derivative (alkylamide) and the elucidation of mechanisms subserving tingle. PLoS One 5:e9520
Atkinson JH PS, Keltner JR (2016). Pharmacologic and non-pharmacologic treatment approaches to chronic pain in individuals with HIV. In: Chronic pain and HIV: a practical approach. Jessica S. Merlin PAS, Glenn J. Treisman, Angela G. Giovanniello, (ed). John Wiley & Sons-Blackwell Publishing Ltd: Hoboken, NJ, pp 97–112
Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV (2011) Brain morphological signatures for chronic pain. PLoS One 6:e26010
Benevides ML, Filho SB, Debona R, Bergamaschi EN, Nunes JC (2017) Prevalence of peripheral neuropathy and associated factors in HIV-infected patients. J Neurol Sci 375:316–320
Beran R (2015) Paraesthesia and peripheral neuropathy. Aust Fam Physician 44:92–95
Berger D (2014) Leg discomfort: beyond the joints. Med Clin North Am 98:429–444
Bilgrami M, O'Keefe P (2014) Neurologic diseases in HIV-infected patients. Handb Clin Neurol 121:1321–1344
Boland EG, Selvarajah D, Hunter M, Ezaydi Y, Tesfaye S, Ahmedzai SH, Snowden JA, Wilkinson ID (2014) Central pain processing in chronic chemotherapy-induced peripheral neuropathy: a functional magnetic resonance imaging study. PLoS One 9:e96474
Brigo F, Tomelleri G, Bovi P, Bovi T (2012) Hemiparesthesias in lacunar pontine ischemic stroke. Neurol Sci 33:619–621
Caplan LR, DeWitt LD, Pessin MS, Gorelick PB, Adelman LS (1988) Lateral thalamic infarcts. Arch Neurol 45:959–964
Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK (2004) Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 26:307–319
Casseb RF, de Paiva JL, Branco LM, Martinez AR, Reis F, de Lima-Junior JC, Castellano G, Junior MC (2016) Spinal cord diffusion tensor imaging in patients with sensory neuronopathy. Neuroradiology 58:1103–1108
Cauda F, Sacco K, Duca S, Cocito D, D'Agata F, Geminiani GC, Canavero S (2009) Altered resting state in diabetic neuropathic pain. PLoS One 4:e4542
Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129:564–583
Chen H, Clifford DB, Deng L, Wu K, Lee AJ, Bosch RJ, Riddler SA, Ellis RJ, Evans SR (2013) Peripheral neuropathy in ART-experienced patients: prevalence and risk factors. J Neuro-Oncol 19:557–564
Cherry CL, McArthur JC, Hoy JF, Wesselingh SL (2003) Nucleoside analogues and neuropathy in the era of HAART. J Clin Virol 26:195–207
Cunningham SI, Tomasi D, Volkow ND (2017) Structural and functional connectivity of the precuneus and thalamus to the default mode network. Hum Brain Mapp 38:938–956
Dorsey SG, Morton PG (2006) HIV peripheral neuropathy: pathophysiology and clinical implications. AACN Clin Issues 17:30–36
Drake CG, McKenzie KG (1953) Mesencephalic tractotomy for pain: experience with six cases. J Neurosurg 10:457–462
Eaton SE, Harris ND, Rajbhandari SM, Greenwood P, Wilkinson ID, Ward JD, Griffiths PD, Tesfaye S (2001) Spinal-cord involvement in diabetic peripheral neuropathy. Lancet 358:35–36
Eldar YC, Oppenheim AV (2003) MMSE whitening and subspace whitening. IEEE trans information theory 49:1846–1851
Ellis RJ, Marquie-Beck J, Delaney P, Alexander T, Clifford DB, McArthur JC, Simpson DM, Ake C, Collier AC, Gelman BB, McCutchan JA, Morgello S, Grant I (2008) Human immunodeficiency virus protease inhibitors and risk for peripheral neuropathy. Ann Neurol 64:566–572
Ellis RJ, Rosario D, Clifford DB, JC MA, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I, Group CS (2010) Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol 67:552–558
Emerson NM, Zeidan F, Lobanov OV, Hadsel MS, Martucci KT, Quevedo AS, Starr CJ, Nahman-Averbuch H, Weissman-Fogel I, Granovsky Y, Yarnitsky D, Coghill RC (2014) Pain sensitivity is inversely related to regional grey matter density in the brain. Pain 155:566–573
Evans SR, Ellis RJ, Chen H, Yeh TM, Lee AJ, Schifitto G, Wu K, Bosch RJ, McArthur JC, Simpson DM, Clifford DB (2011) Peripheral neuropathy in HIV: prevalence and risk factors. AIDS 25:919–928
Feldman EL, Nave KA, Jensen TS, Bennett DL (2017) New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 93:1296–1313
Gonzalez-Duarte A, Robinson-Papp J, Simpson DM (2008) Diagnosis and management of HIV-associated neuropathy. Neurol Clin 26:821–832
Grovle L, Haugen AJ, Keller A, Natvig B, Brox JI, Grotle M (2010) The bothersomeness of sciatica: patients’ self-report of paresthesia, weakness and leg pain. Eur Spine J 19:263–269
Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O (2008) Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159
Henderson KK, Parker J, Heinking KP (2014) Mountaineering-induced bilateral plantar paresthesia. J Am Osteopath Assoc 114:549–555
Herrmann DN, McDermott MP, Henderson D, Chen L, Akowuah K, Schifitto G (2004) Epidermal nerve fiber density, axonal swellings and QST as predictors of HIV distal sensory neuropathy. Muscle Nerve 29:420–427
Hsieh PC, Tseng MT, Chao CC, Lin YH, Tseng WY, Liu KH, Chiang MC, Hsieh ST (2015) Imaging signatures of altered brain responses in small-fiber neuropathy: reduced functional connectivity of the limbic system after peripheral nerve degeneration. Pain 156:904–916
Huang P, Sengupta DK (2014) How fast pain, numbness, and paresthesia resolves after lumbar nerve root decompression: a retrospective study of patient’s self-reported computerized pain drawing. Spine (Phila Pa 1976) 39:E529–E536
Jaggi AS, Singh N (2011) Role of different brain areas in peripheral nerve injury-induced neuropathic pain. Brain Res 1381:187–201
Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Wolfson T, Letendre SL, Ellis RJ, Heaton RK, Gamst AC, Franklin DR Jr, Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I (2011) Clinical factors related to brain structure in HIV: the CHARTER study. J Neuro-Oncol 17:248–257
Jones RK (1974) Meralgia paresthetica as a cause of leg discomfort. Can Med Assoc J 111:541–542
Keltner JR, Akkari C, Ellis RJ (2017a). Neurological complications of HIV in the peripheral nervous system. In: Comprehensive textbook of AIDS psychiatry - a paradigm for integrated care. MA Cohen JG, P Volberding, JM Jacobson, S Letendre, (ed). Oxford University Press: New York
Keltner JR, Connolly CG, Vaida F, Jenkinson M, Fennema-Notestine C, Archibald S, Akkari C, Schlein A, Lee J, Wang D, Kim S, Li H, Rennels A, Miller DJ, Kesidis G, Franklin DR, Sanders C, Corkran S, Grant I, Brown GG, Atkinson JH, Ellis RJ, Group C (2017b) HIV distal neuropathic pain is associated with smaller ventral posterior cingulate cortex. Pain Med 18:428–440
Keltner JR, Fennema-Notestine C, Vaida F, Wang D, Franklin DR, Dworkin RH, Sanders C, McCutchan JA, Archibald SL, Miller DJ, Kesidis G, Cushman C, Kim SM, Abramson I, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Corkran S, Cherner M, Duarte NA, Alexander T, Robinson-Papp J, Gelman BB, Simpson DM, Collier AC, Marra CM, Morgello S, Brown G, Grant I, Atkinson JH, Jernigan TL, Ellis RJ, Group C (2014) HIV-associated distal neuropathic pain is associated with smaller total cerebral cortical gray matter. J Neuro-Oncol 20:209–218
Keltner JR, Vaida F, Ellis RJ, Moeller-Bertram T, Fitzsimmons C, Duarte NA, Robinson-Papp J, Dworkin RH, Clifford DB, McArthur JC, Simpson DM, Collier AC, Marra CM, Atkinson JH, Grant I (2012) Health-related quality of life ‘well-being’ in HIV distal neuropathic pain is more strongly associated with depression severity than with pain intensity. Psychosomatics 53:380–386
Kessy ALA, Strimmer K (2018) Optimal whitening and decorrelation. Am Stat 72:309–314
Kim JS, Choi-Kwon S (1999) Sensory sequelae of medullary infarction: differences between lateral and medial medullary syndrome. Stroke 30:2697–2703
Kim JS, Kim HG, Chung CS (1995) Medial medullary syndrome. Report of 18 new patients and a review of the literature. Stroke 26:1548–1552
Kucyi A, Salomons TV, Davis KD (2013) Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci U S A 110:18692–18697
Lee M, Park CH, Chung HK, Kim HJ, Choi Y, Yoo JH, Yoon YC, Hong YB, Chung KW, Choi BO, Lee HW (2017) Cerebral white matter abnormalities in patients with charcot-marie-tooth disease. Ann Neurol 81:147–151
Lee SH, Kim DE, Song EC, Roh JK (2001) Sensory dermatomal representation in the medial lemniscus. Arch Neurol 58:649–651
Lennertz RC, Tsunozaki M, Bautista DM, Stucky CL (2010) Physiological basis of tingling paresthesia evoked by hydroxy-alpha-sanshool. J Neurosci 30:4353–4361
Lim TH, Choi SI, Yoo JI, Choi YS, Lim YS, Sang BH, Bang YS, Kim YU (2016) Thalamic pain misdiagnosed as cervical disc herniation. Korean J Pain 29:119–122
Maeda Y, Kettner N, Holden J, Lee J, Kim J, Cina S, Malatesta C, Gerber J, McManus C, Im J, Libby A, Mezzacappa P, Morse LR, Park K, Audette J, Tommerdahl M, Napadow V (2014) Functional deficits in carpal tunnel syndrome reflect reorganization of primary somatosensory cortex. Brain 137:1741–1752
Maeda Y, Kettner N, Kim J, Kim H, Cina S, Malatesta C, Gerber J, McManus C, Libby A, Mezzacappa P, Mawla I, Morse LR, Audette J, Napadow V (2016) Primary somatosensory/motor cortical thickness distinguishes paresthesia-dominant from pain-dominant carpal tunnel syndrome. Pain 157:1085–1093
Marchettini P, Lacerenza M, Mauri E, Marangoni C (2006) Painful peripheral neuropathies. Curr Neuropharmacol 4:175–181
Maunsell E, Brisson J, Deschenes L (1993) Arm problems and psychological distress after surgery for breast cancer. Can J Surg 36:315–320
McArthur JC, Brew BJ, Nath A (2005) Neurological complications of HIV infection. Lancet Neurol 4:543–555
Meldgaard B, Andersen K, Ahlgren P, Danielsen UT, Sorensen H (1984) Peripheral neuropathy, cerebral atrophy, and intellectual impairment in chronic alcoholics. Acta Neurol Scand 70:336–344
Mogyoros I, Bostock H, Burke D (2000) Mechanisms of paresthesias arising from healthy axons. Muscle Nerve 23:310–320
Monica M. Diaz MD, John R. Keltner, Donald Franklin, Ronald Ellis. (2019). Longitudinal symptomatic outcomes of patients with HIV-associated distal sensory polyneuropathy. In: 10th International Workshop on HIV & Aging
Moore RD, Wong WM, Keruly JC, McArthur JC (2000) Incidence of neuropathy in HIV-infected patients on monotherapy versus those on combination therapy with didanosine, stavudine and hydroxyurea. AIDS 14:273–278
Morgello S, Estanislao L, Simpson D, Geraci A, DiRocco A, Gerits P, Ryan E, Yakoushina T, Khan S, Mahboob R, Naseer M, Dorfman D, Sharp V, Manhattan HIVBB (2004) HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: the Manhattan HIV Brain Bank. Arch Neurol 61:546–551
Nakashima I, Fujihara K, Kimpara T, Okita N, Takase S, Itoyama Y (2001) Linear pontine trigeminal root lesions in multiple sclerosis: clinical and magnetic resonance imaging studies in 5 cases. Arch Neurol 58:101–104
Navarro X, Vivo M, Valero-Cabre A (2007) Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol 82:163–201
Nora DB, Becker J, Ehlers JA, Gomes I (2005) What symptoms are truly caused by median nerve compression in carpal tunnel syndrome? Clin Neurophysiol 116:275–283
Nudelman KN, McDonald BC, Wang Y, Smith DJ, West JD, O'Neill DP, Zanville NR, Champion VL, Schneider BP, Saykin AJ (2016) Cerebral perfusion and gray matter changes associated with chemotherapy-induced peripheral neuropathy. J Clin Oncol 34:677–683
Prazeres LD, Muniz YV, Barros KM, Gerbi ME, Laureano Filho JR (2013) Effect of infrared laser in the prevention and treatment of paresthesia in orthognathic surgery. J Craniofac Surg 24:708–711
Robinson-Papp J, Morgello S, Vaida F, Fitzsimons C, Simpson DM, Elliott KJ, Al-Lozi M, Gelman BB, Clifford D, Marra CM, McCutchan JA, Atkinson JH, Dworkin RH, Grant I, Ellis R (2010) Association of self-reported painful symptoms with clinical and neurophysiologic signs in HIV-associated sensory neuropathy. Pain 151:732–736
Rocca MA, Valsasina P, Fazio R, Previtali SC, Messina R, Falini A, Comi G, Filippi M (2014) Brain connectivity abnormalities extend beyond the sensorimotor network in peripheral neuropathy. Hum Brain Mapp 35:513–526
Roerink SH, de Ridder M, Prins J, Huijbers A, de Wilt HJ, Marres H, Repping-Wuts H, Stikkelbroeck NM, Timmers HJ, Hermus AR, Netea-Maier RT (2013) High level of distress in long-term survivors of thyroid carcinoma: results of rapid screening using the distress thermometer. Acta Oncol 52:128–137
Selvarajah D, Wilkinson ID, Emery CJ, Harris ND, Shaw PJ, Witte DR, Griffiths PD, Tesfaye S (2006) Early involvement of the spinal cord in diabetic peripheral neuropathy. Diabetes Care 29:2664–2669
Selvarajah D, Wilkinson ID, Emery CJ, Shaw PJ, Griffiths PD, Gandhi R, Tesfaye S (2008) Thalamic neuronal dysfunction and chronic sensorimotor distal symmetrical polyneuropathy in patients with type 1 diabetes mellitus. Diabetologia 51:2088–2092
Selvarajah D, Wilkinson ID, Gandhi R, Griffiths PD, Tesfaye S (2011) Microvascular perfusion abnormalities of the thalamus in painful but not painless diabetic polyneuropathy: a clue to the pathogenesis of pain in type 1 diabetes. Diabetes Care 34:718–720
Selvarajah D, Wilkinson ID, Maxwell M, Davies J, Sankar A, Boland E, Gandhi R, Tracey I, Tesfaye S (2014) Magnetic resonance neuroimaging study of brain structural differences in diabetic peripheral neuropathy. Diabetes Care 37:1681–1688
Seo JP, Jang SH (2013) Traumatic thalamic injury demonstrated by diffusion tensor tractography of the spinothalamic pathway. Brain Inj 27:749–753
Simpson DM, Kitch D, Evans SR, McArthur JC, Asmuth DM, Cohen B, Goodkin K, Gerschenson M, So Y, Marra CM, Diaz-Arrastia R, Shriver S, Millar L, Clifford DB (2006) HIV neuropathy natural history cohort study: assessment measures and risk factors. Neurology 66:1679–1687
Singleton JR (2005) Evaluation and treatment of painful peripheral polyneuropathy. Semin Neurol 25:185–195
Skopelitis E, Aroni K, Kontos AN, Konstantinou K, Kokotis P, Karandreas N, Kordossis T (2007) Early detection of subclinical HIV sensory polyneuropathy using intraepidermal nerve fibre density quantification: association with HIV stage and surrogate markers. Int J STD AIDS 18:856–860
Sorensen L, Siddall PJ, Trenell MI, Yue DK (2008) Differences in metabolites in pain-processing brain regions in patients with diabetes and painful neuropathy. Diabetes Care 31:980–981
Tesfaye S, Selvarajah D, Gandhi R, Greig M, Shillo P, Fang F, Wilkinson ID (2016) Diabetic peripheral neuropathy may not be as its name suggests: evidence from magnetic resonance imaging. Pain 157(Suppl 1):S72–S80
Thomas AG, Dennis A, Bandettini PA, Johansen-Berg H (2012) The effects of aerobic activity on brain structure. Front Psychol 3:86
Tseng MT, Chiang MC, Chao CC, Tseng WY, Hsieh ST (2013) fMRI evidence of degeneration-induced neuropathic pain in diabetes: enhanced limbic and striatal activations. Hum Brain Mapp 34:2733–2746
Verma A (2001) Epidemiology and clinical features of HIV-1 associated neuropathies. J Peripher Nerv Syst 6:8–13
Vilela Filho O (1996) Risk factors for unpleasant paresthesiae induced by paresthesiae-producing deep brain stimulation. Arq Neuropsiquiatr 54:57–63
Vogt BA, Vogt L, Laureys S (2006) Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage 29:452–466
Wang WW, Song CL, Huang L, Song QW, Liang ZH, Wei Q, Hu JN, Miao YW, Wu B, Xie L (2015) DTI study of cerebral normal-appearing white matter in hereditary neuropathy with liability to pressure palsies (HNPP). Medicine (Baltimore) 94:e1909
Wiebe LA, Phillips TJ, Li JM, Allen JA, Shetty K (2011) Pain in HIV: an evolving epidemic. J Pain 12:619–624
Zatorre RJ, Fields RD, Johansen-Berg H (2012) Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 15:528–536
Zhou L, Kitch DW, Evans SR, Hauer P, Raman S, Ebenezer GJ, Gerschenson M, Marra CM, Valcour V, Diaz-Arrastia R, Goodkin K, Millar L, Shriver S, Asmuth DM, Clifford DB, Simpson DM, JC MA, Narc, Group AAS (2007) Correlates of epidermal nerve fiber densities in HIV-associated distal sensory polyneuropathy. Neurology 68:2113–2119
Acknowledgments
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with the Johns Hopkins University, Mount Sinai School of Medicine, University of California, San Diego, University of Texas, Galveston, University of Washington, Seattle, Washington University, St. Louis and is headquartered at the University of California, San Diego and includes: Director: Igor Grant, M.D.; Co-Directors: J. Allen McCutchan, M.D., Ronald J. Ellis, M.D., Ph.D., Thomas D. Marcotte, Ph.D.; Center Manager: Donald Franklin, Jr.; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Terry Alexander, R.N.; Laboratory, Pharmacology and Immunology Component: Scott Letendre, M.D. (P.I.), Edmund Capparelli, Pharm.D.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Matthew Dawson; Virology Component: David Smith, M.D. (P.I.); Imaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Terry L., Jernigan, Ph.D., Michael J. Taylor, Ph.D., Rebecca J. Theilmann, Ph.D., John Hesselink, M.D.; Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D.; Protocol Coordinating Component: Thomas D. Marcotte, Ph.D. (P.I.); Johns Hopkins University Site: Justin McArthur (P.I.), Mary Smith; Mount Sinai School of Medicine Site: Susan Morgello, M.D. (Co-P.I.) and David Simpson, M.D. (Co-P.I.), Letty Mintz, N.P., Cheuk Tang, Ph.D., and Thomas Naidich, M.D.; University of California, San Diego Site: J. Allen McCutchan, M.D. (P.I.), Will Toperoff, N.P.; University of Washington, Seattle Site: Ann Collier, M.D. (Co-P.I.) and Christina Marra, M.D. (Co-P.I.), Kenneth Maravilla, MD, KC Stegbauer, Ph.D., Trudy Jones, M.N., A.R.N.P.; University of Texas, Galveston Site: Benjamin Gelman, M.D., Ph.D. (P.I.), Eleanor Head, R.N., B.S.N., Gregory Chaljub, M.D.; and Washington University, St. Louis Site: David Clifford, M.D. (P.I.), Muhammad Al-Lozi, M.D., Mengesha Teshome, M.D.
Funding
This research was supported by awards 5K23NS079311-03, N01MH22005, R25-MH081482, R01MH079752, P50DA026306, and P30 MH0625 from the National Institutes of Health and HHSN271201000027C from the Department of Health and Human Services and National Institute for Health Research and Oxford Biomedical Research Centre.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
The Human Subjects Protection Committees of each participating institution approved these procedures. Written informed consent was obtained from all study participants as part of enrollment into the CHARTER MRI sub-study.
Conflict of interest
Mark Jenkinson receives royalties from commercial licensing of FSL—other authors have no conflicts of interest to disclose.
Disclaimer
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Keltner, J.R., Tong, A., Visser, E. et al. Evidence for a novel subcortical mechanism for posterior cingulate cortex atrophy in HIV peripheral neuropathy. J. Neurovirol. 26, 530–543 (2020). https://doi.org/10.1007/s13365-020-00850-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-020-00850-3