Abstract
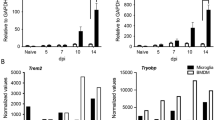
HIV-associated neuroinflammation is primarily driven by CNS macrophages including microglia. Regulation of these immune responses, however, remains to be characterized in detail. Using the SIV/macaque model of HIV, we evaluated CNS expression of triggering receptor expressed on myeloid cells 2 (TREM2) which is constitutively expressed by microglia and contributes to cell survival, proliferation, and differentiation. Loss-of-function mutations in TREM2 are recognized risk factors for neurodegenerative diseases including Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), and Nasu-Hakola disease (NHD); recent reports have also indicated a role for TREM2 in HIV-associated neuroinflammation. Using in situ hybridization (ISH) and qRT-PCR, TREM2 mRNA levels were found to be significantly elevated in frontal cortex of macaques with SIV encephalitis compared with uninfected controls (P = 0.02). TREM2 protein levels were also elevated as measured by ELISA of frontal cortex tissue homogenates in these animals. Previously, we characterized the expression of CSF1R (colony-stimulating factor 1 receptor) in this model; the TREM2 and CSF1R promoters both contain a PU.1 binding site. While TREM2 and CSF1R mRNA levels in the frontal cortex were highly correlated (Spearman R = 0.79, P < 0.001), protein levels were not well correlated. In SIV-infected macaques released from ART to study viral rebound, neither TREM2 nor CSF1R mRNA increased with rebound viremia. However, CSF1R protein levels remained significantly elevated unlike TREM2 (P = 0.02). This differential expression suggests that TREM2 and CSF1R play unique, distinct roles in the pathogenesis of HIV CNS disease.






Similar content being viewed by others
References
Akiyama H, Nishimura T, Kondo H, Ikeda K, Hayashi Y, McGeer PL (1994) Expression of the receptor for macrophage colony stimulating factor by brain microglia and its upregulation in brains of patients with Alzheimer’s disease and amyotrophic lateral sclerosis. Brain Res 639:171–174
Beck SE, Kelly KM, Queen SE, Adams RJ, Zink MC, Tarwater PM, Mankowski JL (2015a) Macaque species susceptibility to simian immunodeficiency virus: increased incidence of SIV central nervous system disease in pigtailed macaques versus rhesus macaques. J Neurovirol 21:148–158. https://doi.org/10.1007/s13365-015-0313-7
Beck SE, Queen SE, Metcalf Pate KA, Mangus LM, Abreu CM, Gama L, Witwer KW, Adams RJ, Zink MC, Clements JE, Mankowski JL (2018) An SIV/macaque model targeted to study HIV-associated neurocognitive disorders. J Neurovirol 24:204–212. https://doi.org/10.1007/s13365-017-0582-4
Beck SE, Queen SE, Witwer KW, Metcalf Pate KA, Mangus LM, Gama L, Adams RJ, Clements JE, Christine Zink M, Mankowski JL (2015b) Paving the path to HIV neurotherapy: predicting SIV CNS disease. Eur J Pharmacol 759:303–312. https://doi.org/10.1016/j.ejphar.2015.03.018
Brenchley JM, Douek DC (2012) Microbial translocation across the GI tract. Annu Rev Immunol 30:149–173. https://doi.org/10.1146/annurev-immunol-020711-075001
Chitu V, Gokhan S, Nandi S, Mehler MF, Stanley ER (2016) Emerging roles for CSF-1 receptor and its ligands in the nervous system. Trends Neurosci 39:378–393. https://doi.org/10.1016/j.tins.2016.03.005
Dardiotis E, Siokas V, Pantazi E, Dardioti M, Rikos D, Xiromerisiou G, Markou A, Papadimitriou D, Speletas M, Hadjigeorgiou GM (2017) A novel mutation in TREM2 gene causing Nasu-Hakola disease and review of the literature. Neurobiol Aging 53:194.e13–194.e22. https://doi.org/10.1016/j.neurobiolaging.2017.01.015
Fields JA, Spencer B, Swinton M, Qvale EM, Marquine MJ, Alexeeva A, Gough S, Soontornniyomkij B, Valera E, Masliah E, Achim CL, Desplats P (2018) Alteration in brain TREM2 and amyloid-beta levels are associated with neurocognitive impairment in HIV-infected persons on antiretroviral therapy. J Neurochem 147:784–802. https://doi.org/10.1111/jnc.14582
Gisslén M, Heslegrave A, Veleva E, Yilmaz A, Andersson LM, Hagberg L, Spudich S, Fuchs D, Price RW, Zetterberg H (2018) CSF concentrations of soluble TREM2 as a marker of microglial activation in HIV-1 infection. Neurol Neuroimmunol Neuroinflamm 6:e512. https://doi.org/10.1212/NXI.0000000000000512
Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, Guan AK, Evans-Reinsch Z, Braz J, Devor M, Abboud-Werner SL, Lanier LL, Lomvardas S, Basbaum AI (2016) Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci 19:94–101. https://doi.org/10.1038/nn.4189
Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J (2013) TREM2 variants in Alzheimer’s disease. N Engl J Med 368:117–127. https://doi.org/10.1056/NEJMoa1211851
Holtman IR, Raj DD, Miller JA, Schaafsma W, Yin Z, Brouwer N, Wes PD, Möller T, Orre M, Kamphuis W, Hol EM, Boddeke EWGM, Eggen BJL (2015) Induction of a common microglia gene expression signature by aging and neurodegenerative conditions: a co-expression meta-analysis. Acta Neuropathol Commun 3:31. https://doi.org/10.1186/s40478-015-0203-5
Irons DL, Meinhardt T, Allers C, Juroda MJ, Kim WK (2019) Overexpression and activation of colony-stimulating factor 1 receptor in the SIV/macaque model of HIV infection and neuroHIV. Brain Pathol 29:826–836. https://doi.org/10.1111/bpa.12731
Jay TR, Miller CM, Cheng PJ, Graham LC, Bemiller S, Broihier ML, Xu G, Margevicius D, Karlo JC, Sousa GL, Cotleur AC, Butovsky O, Bekris L, Staugaitis SM, Leverenz JB, Pimplikar SW, Landreth GE, Howell GR, Ransohoff RM, Lamb BT (2015) TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J Exp Med 212:287–295. https://doi.org/10.1084/jem.20142322
Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K (2013) Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 368:107–116. https://doi.org/10.1056/NEJMoa1211103
Knight AC, Brill SA, Queen SE, Tarwater PM, Mankowski JL (2018) Increased microglial CSF1R expression in the SIV/macaque model of HIV CNS disease. J Neuropathol Exp Neurol 77:199–206. https://doi.org/10.1093/jnen/nlx115
Kobayashi M, Konishi H, Sayo A, Takai T, Kiyama H (2016) TREM2/DAP12 signal elicits proinflammatory response in microglia and exacerbates neuropathic pain. J Neurosci 36:11138–11150. https://doi.org/10.1523/JNEUROSCI.1238-16.2016
Konishi H, Kiyama H (2018) Microglial TREM2/DAP12 signaling: a double-edged sword in neural diseases. Front Cell Neurosci 12. https://doi.org/10.3389/fncel.2018.00206
Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O’Loughlin E, Xu Y, Fanek Z, Greco DJ, Smith ST, Tweet G, Humulock Z, Zrzavy T, Conde-Sanroman P, Gacias M, Weng Z, Chen H, Tjon E, Mazaheri F, Hartmann K, Madi A, Ulrich JD, Glatzel M, Worthmann A, Heeren J, Budnik B, Lemere C, Ikezu T, Heppner FL, Litvak V, Holtzman DM, Lassmann H, Weiner HL, Ochando J, Haass C, Butovsky O (2017) The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 47:566–581.e9. https://doi.org/10.1016/j.immuni.2017.08.008
Laast VA, Shim B, Johanek LM, Dorsey JL, Hauer PE, Tarwater PM, Adams RJ, Pardo CA, McArthur JC, Ringkamp M, Mankowski JL (2011) Macrophage-mediated dorsal root ganglion damage precedes altered nerve conduction in SIV-infected macaques. Am J Pathol 179:2337–2345. https://doi.org/10.1016/j.ajpath.2011.07.047
Otero K, Turnbull IR, Poliani PL, Vermi W, Cerutti E, Aoshi T, Tassi I, Takai T, Stanley SL, Miller M, Shaw AS, Colonna M (2009) Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and β-catenin. Nat Immunol 10:734–743. https://doi.org/10.1038/ni.1744
Satoh J, Asahina N, Kitano S, Kino Y (2014) A comprehensive profile of ChIP-Seq-based PU.1/Spi1 target genes in microglia. Gene Regul Syst Bio. Doi: https://doi.org/10.4137/GRSB.S19711
Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC (2016) HIV-associated neurocognitive disorder – pathogenesis and prospects for treatment. Nat Rev Neurol 12:309. https://doi.org/10.1038/nrneurol.2016.53
Schmid CD, Sautkulis LN, Danielson PE, Cooper J, Hasel KW, Hilbush BS, Sutcliffe JG, Carson MJ (2002) Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J Neurochem 83:1309–1320
Stanley ER, Chitu V (2014) CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol 6. https://doi.org/10.1101/cshperspect.a021857
Thelen M, Razquin C, Hernandez I, Gorostidi A, Sanchez-Valle R, Ortega-Cubero S, Wolfsgruber S, Drichel D, Fliessbach K, Duenkel T, Damian M, Heilmann S, Slotosch A, Lennarz M, Seijo-Martinez M, Rene R, Kornhuber J, Peters O, Luckhaus C, Jahn H, Hull M, Ruther E, Wiltfang J, Lorenzo E, Gascon J, Lleo A, Llado A, Campedalacreu J, Moreno F, Ahmadzadehfar H, Spanish DG, Consortium FJ, Indakoetxea B, Heneka MT, Wetter A, Pastor MA, Riverol M, Becker T, Frolich L, Tarraga L, Boada M, Wagner M, Jessen F, Maier W, Clarimon J, Lopez de Munain A, Ruiz A, Pastor P, Ramirez A (2014) Investigation of the role of rare TREM2 variants in frontotemporal dementia subtypes. Neurobiol Aging 35:2657.e13–2657.e19. https://doi.org/10.1016/j.neurobiolaging.2014.06.018
Uden M, Morley GM, Dibb NJ (1999) Evidence that downregulation of the M-CSF receptor is not dependent upon receptor kinase activity. Oncogene. 18:3846–3851. https://doi.org/10.1038/sj.onc.1202743
Wang Y, Ulland TK, Ulrich JD, Song W, Tzaferis JA, Hole JT, Yuan P, Mahan TE, Shi Y, Gilfillan S, Cella M, Grutzendler S, DeMattos RB, Cirrito JR, Holtzman DM, Colonna M (2016) TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J Exp Med 213:667–675. https://doi.org/10.1084/jem.20151948
Yarandi SS, Peterson DA, Treisman GJ, Moran TH, Pasricha PJ (2016) Modulatory effects of gut microbiota on the central nervous system: how gut could play a role in neuropsychiatric health and disease. J Neurogastroenterol Motil 22:201–212. https://doi.org/10.5056/jnm15146
Yeh FL, Hansen DV, Sheng M (2017) TREM2, microglia, and neurodegenerative diseases. Trends Mol Med 23:512–533. https://doi.org/10.1016/j.molmed.2017.03.008
Acknowledgments
We thank Lisa Mangus, Sarah Beck, Andrew Johanson, and the members of the JHU Retrovirus Laboratory for their contributions. We also thank Drs Feilim MacGabhann, H. Benjamin Larman, and Norman Haughey for their valuable insight and input.
Funding
Funding was provided by NIH R01NS089482, NIH R01NS077869, NIH R01 NS097221, and U42 OD013117. Gilead Sciences, Inc. Foster City, CA, and ViiV Healthcare US, Raleigh, NC, generously donated anti-retroviral compounds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal studies were approved by the Johns Hopkins Animal Care and Use Committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplemental Figure 1
TREM2 ISH colocalizes with IBA1+ CNS macrophages. (A) The double IHC-ISH staining showed that TREM2 ISH (red) colocalizes with IBA1+ cells (brown) in an SIV infected pigtailed macaque with encephalitis. (B) Fluorescence was used to highlight TREM2 ISH staining (red channel). (PNG 3105 kb)
Supplemental Table 1
Study Animals. Description of animals from which samples were taken for the studies described in this paper. (DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Knight, A.C., Brill, S.A., Solis, C.V. et al. Differential regulation of TREM2 and CSF1R in CNS macrophages in an SIV/macaque model of HIV CNS disease. J. Neurovirol. 26, 511–519 (2020). https://doi.org/10.1007/s13365-020-00844-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-020-00844-1