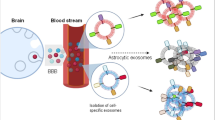
Abstract
Fluid biomarkers for cognitive impairment have the advantage of being relatively noninvasive and capable of monitoring neuronal and other brain cell health in real time. Biomarkers can predict the onset of dementing illness, but also correlate with cognition in a dynamic way allowing us to follow treatment responses and determine brain recovery. Chronic HIV infection causes cognitive impairment in a subset of individuals suggesting “premature aging.” Exosomes are small extracellular vesicles that are shed from all cells. They are important in normal cell-to-cell communication as they contain cellular proteins, mRNA transcripts, and miRNAs. Exosome cargo varies depending on the health of the cell and pathological state; specific proteins/mRNAs and/or miRNAs are present and may serve as biomarkers. Exosomes of variable cellular origin can be isolated from peripheral blood by various methods. Neuron-derived exosomes (NDEs) can be isolated using a precipitation/immunoaffinity approach using antibodies against neuronal cell adhesion molecule L1CAM and the contents queried for central nervous system (CNS) disorders including HIV-associated neurological disorders (HAND) and Alzheimer’s disease (AD). As these studies are recent, numerous questions arise including which neuronal proteins are in NDEs and whether their contents differ in different CNS pathologies or with age. In addition, can the NDE cargo predict as well as diagnose cognitive impairment and could exosomal contents be used as therapeutic biomarkers, or theramarkers, of neuronal recovery from effective treatment? This mini-review will show some new data and review recent studies on NDE from individuals with HIV infection and AD. HIV-associated neurocognitive disorders (HAND) are pathologies seen in a subset of individuals with chronic HIV infection. They belong to the spectrum of neurodegenerative diseases that result in death or dysfunction of neurons with similarities to Alzheimer disease (AD) but also distinctive differences (reviewed (Canet et al., Front Cell Neurosci 12: 307, 2018)). Both disorders are difficult to diagnose without neuropsychological testing and both need new biomarkers to judge progression as well as recovery with treatment. Both disorders involve neuroinflammation and several common targets. AD is associated with aging and HIV is thought to initiate premature aging. In HIV infection, amyloid beta (Aβ), which is deposited in “plaques” in AD, is soluble and its relevance to HIV-associated cognitive impairment is controversial (Achim et al., J Neuroimmune Pharmacol 4: 190–199, 2009; Rempel and Pulliam, AIDS 19: 127–135, 2005). Aβ deposition is required for AD pathological diagnosis, but is not necessarily causative (Barage and Sonawane, Neuropeptides 52: 1–18, 2015; Hardy and Selkoe, Science 297: 353–356, 2002; Morris et al., Acta Neuropathol Commun 2: 135, 2014). Neurofilament light (NF-L) is a surrogate marker in plasma and cerebrospinal fluid (CSF) for neurodegeneration (Abu-Rumeileh et al., Alzheimers Res Ther 10: 3, 2018; Mattsson et al., JAMA Neurol 74: 557–566, 2017) but continues to be a controversial biomarker for both HAND and AD (Gisslen et al., EBioMedicine 3: 135–140, 2016; Kovacs et al., Eur J Neurol 24:1326–e77, 2017; Norgren et al., Brain Res 987: 25–31, 2003; Rolstad et al., J Alzheimers Dis 45: 873–881, 2015; Yilmaz et al., Expert Rev Mol Diagn 17: 761–770, 2017). Blood biomarkers are needed to advance both HAND and AD fields, as blood draws are less costly than neuroimaging and are minimally invasive compared to lumbar punctures required for CSF acquisition. Extracellular vesicles (EVs) are nanoscale membranous vesicles shed from all cells including those of the central nervous system (CNS) and found in all biofluids; they are divided into exosomes (30–150 nm) originating from late endosomes/multivesicular bodies and microvesicles (150–1000 nm) produced through budding of the plasma membrane. Both types of vesicles are implicated in the pathogenesis of neurodegenerative diseases and may provide biomarkers (Bellingham et al., Front Physiol 3: 124, 2012). In this report, we call the vesicles exosomes, since they are the predominant vesicles in our preparations. They are involved in cell-to-cell communication in normal homeostasis and can be carriers of toxic proteins (Aβ, tau) (Sardar Sinha et al., Acta Neuropathol 136: 41–56, 2018) shed by cells as waste or actively secreted in a degenerative process (review Gupta and Pulliam, J Neuroinflammation 11: 68, 2014). The idea that exosomes originating from a specific cell can be recovered in the plasma using cellular surface markers of interest is intriguing. Neuron derived exosomes (NDEs) were first described in 2015 and isolated using antibodies against neural cell adhesion molecules NCAM or L1CAM, after total plasma exosome isolation (Fiandaca et al., Alzheimers Dement 11: 600–607 e1, 2015). Characterization of NDEs follows guidelines endorsed by the International Society for Extracellular Vesicles and includes Nanoparticle Tracking Analysis (NTA) to determine EV concentration and average diameter; Western Blots for EV markers; ELISAs for neuronal proteins and transmission EM for visualization (Sun et al., AIDS 31: F9–F17, 2017; Tang et al., FASEB J 30: 3097–106, 2016). This innovative isolation of an exosome sub-population has generated interest in using NDE as biomarkers for neurodegenerative diseases like AD, HAND, traumatic brain injury, posttraumatic stress disorder and more (reviews Agoston et al., Brain Inj 31: 1195–1203, 2017; Gupta and Pulliam, J Neuroinflammation 11: 68, 2014; Hu et al., Cell Death Dis 7: e2481, 2016; Karnati et al., J Neurotrauma, 2018; Osier et al., Mol Neurobiol, 2018). Several biomarkers from plasma NDEs were recently reported by the Pulliam lab to be elevated in general cognitive impairment (Sun et al., AIDS 31: F9–F17, 2017). We review our collective data here on HAND and AD and add to the characterization of plasma NDEs as exciting biomarkers of neurodegeneration.



Similar content being viewed by others
References
Abner EL, Jicha GA, Shaw LM, Trojanowski JQ, Goetzl EJ (2016) Plasma neuronal exosomal levels of Alzheimer’s disease biomarkers in normal aging. Ann Clin Transl Neurol 3:399–403
Abu-Rumeileh S, Capellari S, Stanzani-Maserati M, Polischi B, Martinelli P, Caroppo P, Ladogana A, Parchi P (2018) The CSF neurofilament light signature in rapidly progressive neurodegenerative dementias. Alzheimers Res Ther 10:3
Achim CL, Adame A, Dumaop W, Everall IP, Masliah E, Neurobehavioral Research C (2009) Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J NeuroImmune Pharmacol 4:190–199
Agoston DV, Shutes-David A, Peskind ER (2017) Biofluid biomarkers of traumatic brain injury. Brain Inj 31:1195–1203
Anderson AM, Easley KA, Kasher N, Franklin D, Heaton RK, Zetterberg H, Blennow K, Gisslen M, Letendre SL (2018) Neurofilament light chain in blood is negatively associated with neuropsychological performance in HIV-infected adults and declines with initiation of antiretroviral therapy. J Neuro-Oncol
Barage SH, Sonawane KD (2015) Amyloid cascade hypothesis: pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 52:1–18
Bellingham SA, Guo BB, Coleman BM, Hill AF (2012) Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front Physiol 3:124
Canet G, Dias C, Gabelle A, Simonin Y, Gosselet F, Marchi N, Makinson A, Tuaillon E, Van de Perre P, Givalois L, Salinas S (2018) HIV Neuroinfection and Alzheimer’s disease: similarities and potential links? Front Cell Neurosci 12:307
Daily A, Nath A, Hersh LB (2006) Tat peptides inhibit neprilysin. J Neuro-Oncol 12:153–160
Eitan E, Tosti V, Suire CN, Cava E, Berkowitz S, Bertozzi B, Raefsky SM, Veronese N, Spangler R, Spelta F, Mustapic M, Kapogiannis D, Mattson MP, Fontana L (2017) In a randomized trial in prostate cancer patients, dietary protein restriction modifies markers of leptin and insulin signaling in plasma extracellular vesicles. Aging Cell 16:1430–1433
Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, Abner EL, Petersen RC, Federoff HJ, Miller BL, Goetzl EJ (2015) Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement 11(600–7):e1
Gill J, Mustapic M, Diaz-Arrastia R, Lange R, Gulyani S, Diehl T, Motamedi V, Osier N, Stern RA, Kapogiannis D (2018) Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Inj:1–8
Gisslen M, Price RW, Andreasson U, Norgren N, Nilsson S, Hagberg L, Fuchs D, Spudich S, Blennow K, Zetterberg H (2016) Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine 3:135–140
Goetzl EJ, Abner EL, Jicha GA, Kapogiannis D, Schwartz JB (2018a) Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer’s disease. FASEB J 32:888–893
Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, Carlson OD, Mustapic M, Kapogiannis D (2015a) Low neural exosomal levels of cellular survival factors in Alzheimer’s disease. Ann Clin Transl Neurol 2:769–773
Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, Kapogiannis D (2015b) Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 85:40–47
Goetzl EJ, Kapogiannis D, Schwartz JB, Lobach IV, Goetzl L, Abner EL, Jicha GA, Karydas AM, Boxer A, Miller BL (2016) Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J 30:4141–4148
Goetzl EJ, Nogueras-Ortiz C, Mustapic M, Mullins RJ, Abner EL, Schwartz JB, Kapogiannis D (2018b). Deficient neurotrophic factors of CSPG4-type neural cell exosomes in Alzheimer disease. FASEB J: fj201801001
Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL (2005) Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 19:407–411
Gu Z, Eils R, Schlesner M (2016) Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32:2847–2849
Gupta A, Pulliam L (2014) Exosomes as mediators of neuroinflammation. J Neuroinflammation 11:68
Hamlett ED, Goetzl EJ, Ledreux A, Vasilevko V, Boger HA, LaRosa A, Clark D, Carroll SL, Carmona-Iragui M, Fortea J, Mufson EJ, Sabbagh M, Mohammed AH, Hartley D, Doran E, Lott IT, Granholm AC (2016) Neuronal exosomes reveal Alzheimer's disease biomarkers in Down syndrome. Alzheimers Dement
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356
Hu G, Yang L, Cai Y, Niu F, Mezzacappa F, Callen S, Fox HS, Buch S (2016) Emerging roles of extracellular vesicles in neurodegenerative disorders: focus on HIV-associated neurological complications. Cell Death Dis 7:e2481
Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, Frassetto L, Petersen RC, Miller BL, Goetzl EJ (2015) Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer's disease. FASEB J 29:589–596
Karnati HK, Garcia JH, Tweedie D, Becker RE, Kapogiannis D, Greig NH (2018) Neuronal enriched extracellular vesicle proteins as biomarkers for brain traumatic injury. J Neurotrauma
Kovacs GG, Andreasson U, Liman V, Regelsberger G, Lutz MI, Danics K, Keller E, Zetterberg H, Blennow K (2017) Plasma and cerebrospinal fluid tau and neurofilament concentrations in rapidly progressive neurological syndromes: a neuropathology-based cohort. Eur J Neurol 24:1326–1e77
Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C (2016) Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A 113:E968–E977
Mattsson N, Andreasson U, Zetterberg H, Blennow K, Alzheimer's Disease Neuroimaging I (2017) Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol 74:557–566
Morris GP, Clark IA, Vissel B (2014) Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer’s disease. Acta Neuropathol Commun 2:135
Mustapic M, Eitan E, Werner JK Jr, Berkowitz ST, Lazaropoulos MP, Tran J, Goetzl EJ, Kapogiannis D (2017) Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front Neurosci 11:278
Norgren N, Rosengren L, Stigbrand T (2003) Elevated neurofilament levels in neurological diseases. Brain Res 987:25–31
Osier N, Motamedi V, Edwards K, Puccio A, Diaz-Arrastia R, Kenney K, Gill J (2018) Exosomes in acquired neurological disorders: new insights into pathophysiology and treatment. Mol Neurobiol 55:9280–9293
Peterson J, Gisslen M, Zetterberg H, Fuchs D, Shacklett BL, Hagberg L, Yiannoutsos CT, Spudich SS, Price RW (2014) Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PLoS One 9:e116081
Rempel HC, Pulliam L (2005) HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS 19:127–135
Rolstad S, Berg AI, Eckerstrom C, Johansson B, Wallin A (2015) Differential impact of neurofilament light subunit on cognition and functional outcome in memory clinic patients with and without vascular burden. J Alzheimers Dis 45:873–881
Sardar Sinha M, Ansell-Schultz A, Civitelli L, Hildesjo C, Larsson M, Lannfelt L, Ingelsson M, Hallbeck M (2018) Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol 136:41–56
Sun B, Dalvi P, Abadjian L, Tang N, Pulliam L (2017) Blood neuron-derived exosomes as biomarkers of cognitive impairment in HIV. AIDS 31:F9–F17
Tang N, Sun B, Gupta A, Rempel H, Pulliam L (2016) Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-kappaB in endothelial cells. FASEB J 30:3097–3106
Winston CN, Goetzl EJ, Akers JC, Carter BS, Rockenstein EM, Galasko D, Masliah E, Rissman RA (2016) Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst) 3:63–72
Yilmaz A, Blennow K, Hagberg L, Nilsson S, Price RW, Schouten J, Spudich S, Underwood J, Zetterberg H, Gisslen M (2017) Neurofilament light chain protein as a marker of neuronal injury: review of its use in HIV-1 infection and reference values for HIV-negative controls. Expert Rev Mol Diagn 17:761–770
Acknowledgments
We thank Dr. Nicole Fernandes for technical support. We thank the National NeuroAIDS tissue consortium (NNTC) for the human plasma used in the HIV studies.
Funding
The HIV work was supported by the National Institute of Mental Health, NIH, R21MH112483 (LP). The Alzheimer’s research was supported in part by the Intramural Research Program of the National Institute on Aging, NIH (DK).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pulliam, L., Sun, B., Mustapic, M. et al. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J. Neurovirol. 25, 702–709 (2019). https://doi.org/10.1007/s13365-018-0695-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-018-0695-4