Abstract
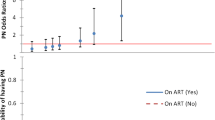
Sensory peripheral neuropathy (PN) remains a common complication in HIV-positive patients despite effective combination anti-retroviral therapy (ART). Data on PN on second-line ART is scarce. We assessed PN using a standard tool in patients failing first-line ART and for 96 weeks following a switch to PI-based second-line ART in a large Randomised Clinical Trial in Sub-Saharan Africa. Factors associated with PN were investigated using logistic regression. Symptomatic PN (SPN) prevalence was 22 % at entry (N = 1,251) and was associated (p < 0.05) with older age (OR = 1.04 per year), female gender (OR = 1.64), Tuberculosis (TB; OR = 1.86), smoking (OR = 1.60), higher plasma creatinine (OR = 1.09 per 0.1 mg/dl increase), CD4 count (OR = 0.83 per doubling) and not consuming alcohol (OR = 0.55). SPN prevalence decreased to 17 % by week 96 (p = 0.0002) following similar trends in all study groups (p = 0.30). Asymptomatic PN (APN) increased over the same period from 21 to 29 % (p = 0.0002). Signs suggestive of PN (regardless of symptoms) returned to baseline levels by week 96. At weeks 48 and 96, after adjusting for time-updated associations above and baseline CD4 count and viral load, SPN was strongly associated with TB (p < 0.0001). In summary, SPN prevalence was significantly reduced with PI-based second-line therapy across all treatment groups, but we did not find any advantage to the NRTI-free regimens. The increase of APN and stability of PN-signs regardless of symptoms suggest an underlying trend of neuropathy progression that may be masked by reduction of symptoms accompanying general health improvement induced by second-line ART. SPN was strongly associated with isoniazid given for TB treatment.


Similar content being viewed by others
References
Arenas-Pinto A, Bhaskaran K, Dunn D, Weller IV (2008) The risk of developing peripheral neuropathy induced by nucleoside reverse transcriptase inhibitors decreases over time: evidence from the Delta trial. Antivir Ther 13(2):289–295
Biraguma J, Rhoda A (2012) Peripheral neuropathy and quality of life of adults living with HIV/AIDS in the Rulindo district of Rwanda. SAHARA J 9(2):88–94
Breen RA, Miller RF, Gorsuch T, Smith CJ, Schwenk A, Holmes W et al (2006) Adverse events and treatment interruption in tuberculosis patients with and without HIV co-infection. Thorax 61(9):791–794
Cettomai D, Kwasa J, Kendi C, Birbeck GL, Price RW, Bukusi EA et al (2010) Utility of quantitative sensory testing and screening tools in identifying HIV-associated peripheral neuropathy in Western Kenya: pilot testing. PLoS One 5(12):e14256
Chen H, Clifford DB, Deng L, Wu K, Lee AJ, Bosch RJ et al (2013) Peripheral neuropathy in ART-experienced patients: prevalence and risk factors. J Neurovirol 19(6):557–564
Cherry CL, Affandi JS, Imran D, Yunihastuti E, Smyth K, Vanar S et al (2009) Age and height predict neuropathy risk in patients with HIV prescribed stavudine. Neurology 73(4):315–320
Childs EA, Lyles RH, Selnes OA, Chen B, Miller EN, Cohen BA et al (1999) Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology 52(3):607–613
Collins MA, Neafsey EJ, Zou JY (2000) HIV-I gpI20 neurotoxicity in brain cultures is prevented by moderate ethanol pretreatment. Neuroreport 11(6):1219–1222
Dean GL, Edwards SG, Ives NJ, Matthews G, Fox EF, Navaratne L et al (2002) Treatment of tuberculosis in HIV-infected persons in the era of highly active antiretroviral therapy. AIDS 16(1):75–83
Ellis RJ, Evans SR, Clifford DB, Moo LR, McArthur JC, Collier AC et al (2005) Clinical validation of the NeuroScreen. J Neurovirol 11(6):503–511
Ellis RJ, Marquie-Beck J, Delaney P, Alexander T, Clifford DB, McArthur JC et al (2008) Human immunodeficiency virus protease inhibitors and risk for peripheral neuropathy. Ann Neurol 64(5):566–572
Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T et al (2010) Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol 67(5):552–558
Evans SR, Ellis RJ, Chen H, Yeh TM, Lee AJ, Schifitto G et al (2011) Peripheral neuropathy in HIV: prevalence and risk factors. AIDS 25(7):919–928
Evans D, Takuva S, Rassool M, Firnhaber C, Maskew M (2012) Prevalence of peripheral neuropathy in antiretroviral therapy naive HIV-positive patients and the impact on treatment outcomes—a retrospective study from a large urban cohort in Johannesburg, South Africa. J Neurovirol 18(3):162–171
Grant AD, Mngadi KT, van Halsema CL, Luttig MM, Fielding KL, Churchyard GJ (2010) Adverse events with isoniazid preventive therapy: experience from a large trial. AIDS 24(Suppl 5):S29–36
Kamerman PR, Wadley AL, Cherry CL (2012) HIV-associated sensory neuropathy: risk factors and genetics. Curr Pain Headache Rep 16(3):226–236
Kampira E, Kumwenda J, van Oosterhout JJ, Dandara C (2013) Mitochondrial DNA subhaplogroups L0a2 and L2a modify susceptibility to peripheral neuropathy in malawian adults on stavudine containing highly active antiretroviral therapy. J Acquir Immune Defic Syndr 63(5):647–652
Lichtenstein KA, Armon C, Baron A, Moorman AC, Wood KC, Holmberg SD (2005) Modification of the incidence of drug-associated symmetrical peripheral neuropathy by host and disease factors in the HIV outpatient study cohort. Clin Infect Dis 40(1):148–157
Luma HN, Tchaleu BC, Doualla MS, Temfack E, Sopouassi VN, Mapoure YN et al (2012) HIV-associated sensory neuropathy in HIV-1 infected patients at the Douala General Hospital in Cameroon: a cross-sectional study. AIDS Res Ther 9(1):35
Maritz J, Benatar M, Dave JA, Harrison TB, Badri M, Levitt NS et al (2010) HIV neuropathy in South Africans: frequency, characteristics, and risk factors. Muscle Nerve 41(5):599–606
Marra CM, Boutin P, Collier AC (1998) Screening for distal sensory peripheral neuropathy in HIV-infected persons in research and clinical settings. Neurology 51(6):1678–1681
Mehta SA, Ahmed A, Laverty M, Holzman RS, Valentine F, Sivapalasingam S (2011) Sex differences in the incidence of peripheral neuropathy among Kenyans initiating antiretroviral therapy. Clin Infect Dis 53(5):490–496
Nakamoto BK, McMurtray A, Davis J, Valcour V, Watters MR, Shiramizu B et al (2010) Incident neuropathy in HIV-infected patients on HAART. AIDS Res Hum Retrovir 26(7):759–765
Paik IJ, Kotler DP (2011) The prevalence and pathogenesis of diabetes mellitus in treated HIV-infection. Best Pract Res Clin Endocrinol Metab 25(3):469–478
Paton NI, Kityo C, Hoppe A, Reid A, Kambugu A, Lugemwa A et al (2014) Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med 371(3):234–247
Pettersen JA, Jones G, Worthington C, Krentz HB, Keppler OT, Hoke A et al (2006) Sensory neuropathy in human immunodeficiency virus/acquired immunodeficiency syndrome patients: protease inhibitor-mediated neurotoxicity. Ann Neurol 59(5):816–824
Shaikh A, Bentley A, Kamerman PR (2013) Symptomatology of peripheral neuropathy in an African language. PLoS One 8(5):e63986
van Buuren S, Boshuizen HC, Knook DL (1999) Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 18(6):681–694
van der Watt JJ, Harrison TB, Benatar M, Heckmann JM (2011) Polyneuropathy, anti-tuberculosis treatment and the role of pyridoxine in the HIV/AIDS era: a systematic review. Int J Tuberc Lung Dis 15(6):722–728
van Oosterhout JJ, Mallewa J, Kaunda S, Chagoma N, Njalale Y, Kampira E et al (2012) Stavudine toxicity in adult longer-term ART patients in Blantyre, Malawi. PLoS One 7(7):e42029
Wadley AL, Cherry CL, Price P, Kamerman PR (2011) HIV neuropathy risk factors and symptom characterization in stavudine-exposed South Africans. J Pain Symptom Manag 41(4):700–706
Westreich DJ, Sanne I, Maskew M, Malope-Kgokong B, Conradie F, Majuba P et al (2009) Tuberculosis treatment and risk of stavudine substitution in first-line antiretroviral therapy. Clin Infect Dis 48(11):1617–1623
Wright E, Brew B, Arayawichanont A, Robertson K, Samintharapanya K, Kongsaengdao S et al (2008) Neurologic disorders are prevalent in HIV-positive outpatients in the Asia-Pacific region. Neurology 71(1):50–56
Acknowledgments
We thank all the patients and staff from all the centres participating in the EARNEST trial. The EARNEST trial was funded by the European and Developing Countries Clinical Trials Partnership (EDCTP) with contributions from the Medical Research Council, UK, Institito de Salud Carlos III, Spain, Irish Aid, Ireland, Swedish International Development Cooperation Agency (SIDA), Sweden, Instituto Superiore di Sanita (ISS), Italy and Merck, USA. Substantive in-kind contributions were made by the Medical Research Council Clinical Trials Unit, UK, CINECA, Bologna, Italy, Janssen Diagnostics, Mechelen, Belgium; GSK, UK; Abbott Laboratories, USA. Trial medication was donated by AbbVie, Merck, Pfizer, GSK and Gilead
Conflict of interest
NIP was the EDCTP grant recipient for this trial. NIP, ASW, MT, JT and AAP are employed by the MRC-UK. NIP, ASW and AAP have received funding support for other studies from GSK or Janssen. AM, GM and HM have received support from the Research Councils-UK for research projects. NIP has received payments for lectures from Merck, Janssen and AbbVie. AAP has received payments for lectures from Janssen. Institutional payment has been received from Gilead Sciences because of a lecture given by ASW. ASW has been DSMB member—for studies sponsored by Tibotec.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Members of the Europe Africa Research Network for Evaluation of Second-line Therapy (EARNEST) Trial Team are listed in the Appendix
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 85 kb)
The EARNEST Trial Team are:
The EARNEST Trial Team are:
Participating Sites
Uganda:
JCRC Kampala (African trial co-ordinating centre; 231) E Agweng, P Awio, G Bakeinyaga, C Isabirye, U Kabuga, S Kasuswa, M Katuramu, C Kityo, F Kiweewa, H Kyomugisha, E Lutalo, P Mugyenyi, D Mulima, H Musana, G Musitwa, V Musiime, M Ndigendawan, H Namata, J Nkalubo, P Ocitti Labejja, P Okello, P Olal, G Pimundu, P Segonga, F Ssali, Z Tamale, D Tumukunde, W Namala, R Byaruhanga, J Kayiwa, J Tukamushaba.
IDI, Kampala (216): G Bihabwa, E Buluma, P Easterbrook, A Elbireer, A Kambugu, D Kamya, M Katwere, R Kiggundu, C Komujuni, E Laker, E Lubwama, I Mambule, J Matovu, A Nakajubi, J Nakku, R Nalumenya, L Namuyimbwa, F Semitala, B Wandera, J Wanyama
JCRC, Mbarara (97): H Mugerwa, A Lugemwa, E Ninsiima, T Ssenkindu, S Mwebe, L Atwine, H William, C Katemba, S Abunyang, M Acaku, P Ssebutinde, H Kitizo, J Kukundakwe, M Naluguza, K Ssegawa, Namayanja, F Nsibuka, P Tuhirirwe, M Fortunate
JCRC Fort Portal (66): J Acen, J Achidri, A Amone, M. Chamai, J Ditai, M Kemigisa, M Kiconco, C Matama, D Mbanza, F Nambaziira, M Owor Odoi, A Rweyora, G. Tumwebaze
San Raphael of St Francis Hospital, Nsambya (48): H Kalanzi, J Katabaazi , A Kiyingi, M Mbidde, M. Mugenyi, R Mwebaze, P Okong, I Senoga
JCRC Mbale (47): M Abwola, D Baliruno, J Bwomezi, A Kasede, M Mudoola, R Namisi, F Ssennono, S Tuhirwe
JCRC Gulu (43): G Abongomera, G Amone, J Abach, I Aciro, B Arach, P Kidega, J Omongin, E Ocung, W Odong, A Philliam
JCRC Kabale (33): H Alima, B Ahimbisibwe, E Atuhaire, F Atukunda, G Bekusike, A Bulegyeya, D. Kahatano, S Kamukama, J Kyoshabire, A Nassali, A Mbonye, T M Naturinda, Ndukukire, A Nshabohurira, H. Ntawiha, A Rogers, M Tibyasa;
JCRC Kakira (31): S. Kiirya, D. Atwongeire, A. Nankya, C. Draleku, D. Nakiboneka, D. Odoch, L. Lakidi, R. Ruganda, R. Abiriga, M. Mulindwa, F. Balmoi, S. Kafuma, E. Moriku
Zimbabwe
University of Zimbabwe Clinical Research Centre, Harare (265): J Hakim, A Reid, E Chidziva, GMusoro,
C Warambwa, G Tinago, S Mutsai, M Phiri, S Mudzingwa, T Bafana, V Masore, C Moyo, R Nhema, S Chitongo.
Malawi
College of Medicine, University of Malawi, Blanytre (92): Rob Heyderman, Lucky Kabanga, Symon Kaunda, Aubrey Kudzala, Linly Lifa, Jane Mallewa, Mike Moore, Chrissie Mtali, George Musowa, Grace Mwimaniwa, Rosemary Sikwese, Joep van Oosterhout, Milton Ziwoya
Mzuzu Central Hospital, Mzuzu (19): H Chimbaka. B Chitete, S Kamanga, T Kayinga E Makwakwa, R Mbiya, M Mlenga, T Mphande, C Mtika, G Mushani, O Ndhlovu, M Ngonga, I Nkhana, R Nyirenda
Kenya
Moi Teaching and Referral Hospital (52): P Cheruiyot, C Kwobah, W Lokitala Ekiru, M Mokaya, A Mudogo, A Nzioka, A Siika, M Tanui, S Wachira, K Wools-Kaloustian
Zambia
University Teaching Hospital (37): P Alipalli, E Chikatula, J Kipaila, I Kunda, S Lakhi, J Malama, W Mufwambi, L Mulenga, P Mwaba, E Mwamba, A Mweemba, M Namfukwe
The Aids Support Organisation (TASO), Uganda: E Kerukadho, B Ngwatu, J Birungi
MRC Clinical Trials Unit: N Paton, J Boles, A Burke, L Castle, S Ghuman, L Kendall, A Hoppe, S Tebbs, M Thomason, J Thompson, S Walker, J Whittle, H Wilkes, N Young
Monitors: C Kapuya, F Kyomuhendo, D Kyakundi, N Mkandawire, S Mulambo, S Senyonjo
Clinical Expert Review Committee: B Angus, A Arenas-Pinto, A Palfreeman, F Post, D Ishola
European Collaborators: J Arribas, B Colebunders, M Floridia, M Giuliano, P Mallon, P Walsh, M De Rosa, E Rinaldi
Trial Steering Committee: I Weller (Chair), C Gilks, J Hakim, A Kangewende, S Lakhi, E Luyirika, F Miiro, P Mwamba, P Mugyenyi, S Ojoo, N Paton, S Phiri, J van Oosterhout, A Siika, S Walker, A Wapakabulo,
Data Monitoring Committee: T Peto (Chair), N French, J Matenga
Pharmaceutical companies: G Cloherty, J van Wyk, M Norton, S Lehrman, P Lamba, K Malik, J Rooney, W Snowden, J Villacian
Rights and permissions
About this article
Cite this article
Arenas-Pinto, A., Thompson, J., Musoro, G. et al. Peripheral neuropathy in HIV patients in sub-Saharan Africa failing first-line therapy and the response to second-line ART in the EARNEST trial. J. Neurovirol. 22, 104–113 (2016). https://doi.org/10.1007/s13365-015-0374-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-015-0374-7