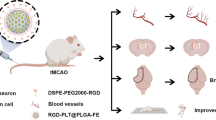
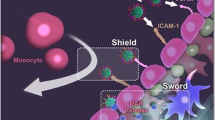
Abstract
Microglia are important cells that act on regulating neuroinflammation and neurofunction after the induction of ischemic stroke (IS). Consequently, the efficient accumulation of drugs within ischemic regions, particularly in microglia, serves as a valuable approach for achieving effective therapy by attenuating microglia-mediated cerebral ischemic injury. In this study, we designed mannose (man)-conjugated luteolin (lut)-loaded platelet-derived exosomes (lut/man-pEXO) as surface engineered multifunctional cascade-delivery drug carriers to target ischemic blood vessels and subsequent microglia to enhance drug accumulation and induce neuroprotection of neurovascular unit (NVU) against IS. The results revealed that as platelets naturally gathered in pathological ischemic cerebral vessels, lut/man-pEXO could bind to platelets and efficiently target ischemic injury sites. Moreover, owing to the selective binding affinity of mannose present in lut/man-pEXO towards the mannose receptor expressed on microglia, lut/man-pEXO exhibited superior microglia-targeting properties, inducing the increased uptake of lut by microglia. As a result, lut/man-pEXO regulated microglia by inhibiting the activation of detrimental M1 and promoting the transition towards the anti-inflammatory type (M2), thus attenuating ischemic damage of NVU by reducing the infarct area, rescuing the damage of blood–brain barrier (BBB) and preventing inflammatory transformation of astrocytes.
Graphical Abstract








Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Lo EH, Broderick JP, Moskowitz MA. tPA and proteolysis in the neurovascular unit. Stroke. 2004;35(2):354–6.
Papanagiotou P, White CJ. Endovascular reperfusion strategies for acute stroke. JACC Cardiovasc Interv. 2016;9(4):307–17.
Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther. 2013;138(2):155–75.
Pan W, Banks WA, Kastin AJ. Permeability of the blood-brain barrier to neurotrophins. BrainRes. 1998;788(1–2):87–94.
Muoio V, Persson PB, Sendeski MM. The neurovascular unit - concept review. Acta Physiol (Oxf). 2014;210(4):790–8.
Lu Y, Li C, Chen Q, et al. Microthrombus-targeting micelles for neurovascular remodeling and enhanced microcirculatory perfusion in acute ischemic stroke. Adv Mater. 2019;31(21): e1808361.
Liu LR, Liu JC, Bao JS, Bai QQ, Wang GQ. Interaction of microglia and astrocytes in the neurovascular unit. Front Immunol. 2020;11:1024.
Hu X, Leak RK, Shi Y, et al. Microglial and macrophage polarization—new prospects for brain repair. Nat Rev Neurol. 2015;11(1):56–64.
Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are indued byactivate dmicroglia. Nature. 2017;541(7638):481–7.
Quintas C, Pinho D, Pereira C, Saraiva L, Gonçalves J, Queiroz G. Microglia P2Y6 receptors mediate nitric oxide release and astrocyte apoptosis. J Neuroinflammation. 2014;11:141.
Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006;37(4):1087–93.
Wang D, Li SP, Fu JS, Zhang S, Bai L, Guo L. Resveratrol defends blood-brain barrier integrity in experimental autoimmune encephalomyelitis mice. J Neurophysiol. 2016;116(5):2173–9.
Ma Y, Wang J, Wang Y, Yang GY. The biphasic function of microglia in ischemic stroke. ProgNeurobiol. 2017;157:247–272.
Vay SU, Flitsch LJ, Rabenstein M, et al. The plasticity of primary microglia and their multifaceted effects on endogenous neural stem cells in vitro and in vivo. J Neuroinflammation. 2018;15(1):226.
Nabavi SF, Braidy N, Gortzi O, et al. Luteolin as an anti-inflammatory and neuroprotective agent: a brief review. Brain Res Bull. 2015;119(Pt A):1–11.
Kempuraj D, Thangavel R, Kempuraj DD, et al. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. BioFactors. 2021;47(2):190–7.
Yao ZH, Yao XL, Zhang Y, Zhang SF, Hu JC. Luteolin could improve cognitive dysfunction by inhibiting neuroinflammation. Neurochem Res. 2018;43(4):806–20.
Liu X, Zhang M, Tian Y, et al. Development, characterization, and investigation of in vivo targeted delivery efficacy of luteolin-loaded, eudragit S100-coated mPEG-PLGA nanoparticles. AAPS PharmSciTech. 2022;23(4):100.
Hu Y, Chen X, Li Z, Zheng S, Cheng Y. Thermosensitive in situ gel containing luteolin micelles is a promising efficient agent for colorectal cancer peritoneal metastasis treatment. J Biomed Nanotechnol. 2020;16(1):54–64.
Ma Y, Zhang Y, Han R, et al. A cascade synergetic strategy induced by photothermal effect based on platelet exosome nanoparticles for tumor therapy. Biomaterials. 2022;282: 121384.
Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–9.
Yu W, Yin N, Yang Y, et al. Rescuing ischemic stroke by biomimetic nanovesicles through accelerated thrombolysis and sequential ischemia-reperfusion protection. Acta Biomater. 2022;140:625–40.
Xu J, Wang X, Yin H, et al. Sequentially site-specific delivery of thrombolytics and neuroprotectant for enhanced treatment of ischemic stroke. ACS Nano. 2019;13(8):8577–88.
Zhang Y, Wang X, Chen J, et al. Exosomes derived from platelet-rich plasma administration in site mediate cartilage protection in subtalar osteoarthritis. J Nanobiotechnology. 2022;20(1):56.
Li Y, Teng X, Yang C, et al. Ultrasound controlled anti-inflammatory polarization of platelet decorated microglia for targeted ischemic stroke therapy. Angew Chem Int Ed Engl. 2021;60(10):5083–90.
Qi Y, Guo L, Jiang Y, Shi Y, Sui H, Zhao L. Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Deliv. 2020;27(1):745–55.
Choi ES, Song J, Kang YY, Mok H. Mannose-modified serum exosomes for the elevated uptake to murine dendritic cells and lymphatic accumulation. Macromol Biosci. 2019;19(7): e1900042.
Liu W, Su C, Qi Y, Liang J, Zhao L, Shi Y. Brain-targeted heptapeptide-loaded exosomes attenuated ischemia-reperfusion injury by promoting the transfer of healthy mitochondria from astrocytes to neurons. J Nanobiotechnology. 2022;20(1):242.
Zheng Y, He R, Wang P, Shi Y, Zhao L, Liang J. Exosomes from LPS-stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization. Biomater Sci. 2019;7(5):2037–49.
Su C, Wang Q, Zhang H, et al. Si-Miao-Yong-An decoction protects against cardiac hypertrophy and dysfunction by inhibiting platelet aggregation and activation. Front Pharmacol. 2019;10:990.
Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–70.
Haseloff RF, Dithmer S, Winkler L, Wolburg H, Blasig IE. Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects. Semin Cell Dev Biol. 2015;38:16–25.
Chen X, Nie X, Mao J, Zhang Y, Yin K, Jiang S. Perfluorooctanesulfonate induces neuroinflammation through the secretion of TNF-α mediated by the JAK2/STAT3 pathway. Neurotoxicology. 2018;66:32–42.
Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4(5):399–415.
Ginsberg MD. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke. 2009;40(3Suppl):S111-S114.
O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59(3):467–77.
Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008;9(3):169–81.
del Zoppo GJ. The neurovascular unit in the setting of stroke. J Intern Med. 2010;267(2):156–71.
Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13(4):661–70.
Fowler MJ, Cotter JD, Knight BE, Sevick-Muraca EM, Sandberg DI, Sirianni RW. Intrathecal drug delivery in the era of nanomedicine. Adv Drug Deliv Rev. 2020;165–166:77–95.
Ku S, Yan F, Wang Y, Sun Y, Yang N, Ye L. The blood-brain barrier penetration and distribution of PEGylated fluorescein-doped magnetic silica nanoparticles in rat brain. Biochem Biophys Res Commun. 2010;394(4):871–6.
Patel T, Zhou J, Piepmeier JM, Saltzman WM. Polymeric nanoparticles for drug delivery to the central nervous system. Adv Drug Deliv Rev. 2012;64(7):701–5.
Wen CJ, Zhang LW, Al-Suwayeh SA, Yen TC, Fang JY. Theranostic liposomes loaded with quantum dots and apomorphine for brain targeting and bioimaging. Int J Nanomedicine. 2012;7:1599–611.
Zhang P, Hu L, Yin Q, Feng L, Li Y. Transferrin-modified c[RGDfK]-paclitaxel loaded hybrid micelle for sequential blood-brain barrier penetration and glioma targeting therapy. Mol Pharm. 2012;9(6):1590–8.
Lai CP, Mardini O, Ericsson M, et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8(1):483–94.
Acknowledgements
We thank Tao Xu, Hongdan Li, Jia Liang, and Song Zhao from the Life Science Institute of Jinzhou Medical University for their technical support.
Funding
This work was supported by grants of Project of Liaoning Educational Committee (JYTJCZR2020067 and JYTQN2020015). We thank the support of the above funds.
Author information
Authors and Affiliations
Contributions
Conceptualization, C. S. and L. Z.; methodology, X. L.; validation, X. L., Y. H., and Z.H.; formal analysis, X. L. and Y. H.; investigation, X. L. and Y. S.; writing—original draft preparation, X. L.; writing—review and editing, L. Z.; supervision, C. S. and L. Z.; and funding acquisition, Y. S. and L. Z.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Research experiments conducted in this article with animals were approved by the Ethical Committee and responsible authorities of Jinzhou Medical University following all guidelines, regulations, legal, and ethical standards as required for animals and followed the National Guidelines for Animal Protection.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Hao, Y., Huang, Z. et al. Modulation of microglial polarization by sequential targeting surface-engineered exosomes improves therapy for ischemic stroke. Drug Deliv. and Transl. Res. 14, 418–432 (2024). https://doi.org/10.1007/s13346-023-01408-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-023-01408-6