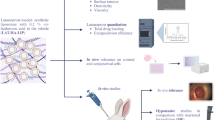
Abstract
Glaucoma is a chronic eye disease in which the pressure inside the eye increases and leads to damage to the optic nerve, and eventually causes blindness. In this disease, it is often necessary to use a multi-drug treatment system. There is a fixed combination of timolol maleate and brimonidine tartrate among the combination drugs in glaucoma treatment. Liposomes are one of the most important targeted drug delivery systems to eye tissue, which leads to improved drug permeability and durability in ocular tissue. In this study, thin layer hydration was used to make liposomal formulations containing timolol maleate (TM) and brimonidine tartrate (BT). After the necessary evaluations, one of the eight initial formulations was selected as an optimization formulation. Then, characteristics such as drug loading percentage, particle size, pH, zeta potential, and drug release were performed on the optimized formulation. The study of reducing intraocular pressure was performed on the optimized formulation. This study in total was performed on 18 rabbits in three groups. Hydroxypropyl methylcellulose (HPMC) polymer was injected into the anterior chamber to experimental induce glaucoma. The selected formulation was within the acceptable range of ocular products in terms of physical properties. HPMC polymer injection successfully induced glaucoma in the animal model, resulting in a 79% increase in intraocular pressure. The results showed that the liposomal formulation significantly reduced the intraocular pressure compared to the simple formulation of the aqueous solution, and both formulations were able to significantly reduce the intraocular pressure compared to the control group (P < 0.001). The results also showed that liposomal formulation has a therapeutic effect in reducing intraocular pressure. It seems that the selected liposomal formulation made by thin layer hydration can act as a suitable drug carrier to increase the effectiveness of the fixed combination of timolol maleate and brimonidine tartrate and be proposed as a new drug formulation for targeted and controlled drug delivery in the treatment of glaucoma.
Graphical Abstract





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma. JAMA. 2014;311(18):1901–1911.
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Omlthalmol. 2006;90:262–7.
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90.
Gan L, Wang J, Jiang M, Bartlett H, Ouyang D, Eperjesi F, et al. Recent advance in topical ophthalmic drug delivery with lipid-based Nano carriers. Drug Discov Today. 2013;18(5–6):290–7.
Del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov Today. 2008;13:135–143.
Xu J, Xue Y, Hu G, Lin T, Gou J, Yin T, He H, Zhang Y, Tang X. A comprehensive review on contact lens for ophthalmic drug delivery. J Control Release. 2018;281:97–118.
Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surg Lasers Imaging Retina. 1995;26(3):233–6.
Kobelt-Nguyen G, Gerdtham UG, Alm A. Costs of treating primary open-angle glaucoma and ocular hypertension: a retrospective, observational two-year chart review of newly diagnosed patients in Sweden and the United States. J Glaucoma. 1998;7(2):95–104.
Weinreb RN. Compliance with medical treatment of glaucoma. J Glaucoma. 1992;1(2):134–6.
Lichter PR, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108(11):1943–53.
Katz LJ. Modern alchemy: fixed combinations of glaucoma drugs. Am j ophthalmol. 2005;1(140):125–26.
Robin AL, Covert D. Does adjunctive glaucoma therapy affect adherence to the initial primary therapy? Ophthalmology. 2005;112(5):863–8.
Holló G, et al. Long-term outcomes of prostaglandin analog versus timolol maleate in ocular hypertensive or primary open-angle glaucoma patients in Europe. J Ocul Pharmacol Ther. 2011;27(5):493–98.
Fechtner RD, Realini T. Fixed combinations of topical glaucoma medications. Curr Opin Ophthalmol. 2004;15(2):132–5.
Samy KE, Cao Y, Kim J, Konichi da Silva NR, Phone A, Bloomer MM, Bhisitkul RB, Desai TA. Co-delivery of timolol and brimonidine with a polymer thin-film intraocular device. J Ocul Pharmacol Ther. 2019;35:124–131.
Frampton JE. Topical brimonidine 0.2%/timolol 0.5% ophthalmic solution. Drugs Aging. 2006;23(9):753–61.
Goni FJ, Brimonidine/Timolol Fixed Combination Study Group. 12-week study comparing the fixed combination of brimonidine and timolol with concomitant use of the individual components in patients with glaucoma and ocular hypertension. Eur J Ophthalmol. 2005;15(5):581–90.
Arcieri ES, Arcieri RS, Pereira ACA, Andreo EGV, Finotti IGA, SáFilho WF. Comparing the fixed combination brimonidine-timolol versus fixed combination dorzolamide-timolol in patients with elevated intraocular pressure. Curr Med Res Opin. 2007;23:683–9.
Schield's. Text book of glaucoma. 5th ed. Chapter 1 Cellular and molecular biology of aqueous humor dynamics. 15–17.
Katzung BG. Basic & clinical pharmacology. section II; chapter 6. Introduction to autonomic pharmacology. Pharmacology of Eye. 91.
Henderer GD, Rapuano CJ. Goodman & Gilmans. Pharmacol Basis Ther. 1722-23.
Yellepeddi VK, Palakurthi S. Recent advances in topical ocular drug delivery. J Ocul Pharmacol Ther. 2016;32(2):67-82.
Honda M, Asai T, Oku N, Araki Y, Tanaka M, Ebihara N. Liposomes and nanotechnology in drug development: focus on ocular targets. Int J Nanomed. 2013;8:495.
Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238–52.
Elshanawane AA, Abdolaziz LM, Mohram MS, Hafez HM. Development and validation of HPLC method for simultaneous estimation of Brimonidine tartrate and timolol maleate in bulk and pharmaceutical dosage forms. J Chromatograph SeparatTechniq. 2014;5:3.
Aggarwal D, Garg A, Kaur IP. Development of a topical niosomal preparation of acetazolamide: preparation and evaluation. J Pharm Pharmacol. 2004;56(12):1509–17.
Maiti S, Paul S, Mondol R, Ray S, Sa B. Nanovesicular formulation of brimonidine tartrate for the management of glaucoma: in vitro and in vivo evaluation. AapsPharmscitech. 2011;12(2):755–63.
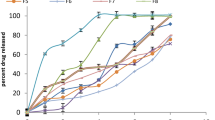
Higuchi T. Mechanism of sustained action medication. Theoretical analysis of rate release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145-49.
Zhu MD, Cai FY. Development of experimental chronic intraocular hypertension in the rabbit. Aust N Z J Ophthalmol. 1992;20(3):225–34.
Kouchak M, Bahmandar R, Bavarsad N, Farrahi F. Ocular DorzolamideNanoliposomes for Prolonged IOP Reduction: in-vitro and in-vivo Evaluation in Rabbits. Iranian J Pharm Res: IJPR. 2016;15(1):205.
Pokharkar V, Patil V, Mandpe L. Engineering of polymer-surfactant nanoparticles of doxycycline hydrochloride for ocular drug delivery. Drug Deliv. 2015;92(7):955–68.
Morand K, Bartoletti AC, Bochot A, Barratt G, Brandely ML, Chast F. Liposomal amphotericin B eye drops to treat fungal keratitis: physico-chemical and formulation stability. Int J Pharm. 2007;344(1–2):150–3.
Hecht G. Ophthalmic preparations. 4th ed. New York: University of Sciences in Philadelphia. 2001.
Kaur IP, Singh M, Kanwar M. Formulation and evaluation of ophthalmic preparations of acetazolamide. Int J Pharm. 2000;199(2):119–27.
USP29-NF24. Pharmaceutical dosage forms, ophthalmic preparation and uniformity of dosage units chapters. In: The United States Pharmacopeia 29th, The National Formulary 24th. Rockville, MD: United States Pharmacopeial Convention, Inc. 2005.
Scholes PD, Coombes AG, Illum L, Daviz SS, Vert M, Davies MC. The preparation of sub-200 nm poly (lactide-co-glycolide) microspheres for site-specific drug delivery. J Control Release. 1993;25(1–2):145–53.
Ibrahim MM, Abd-Elgawad AE, Soliman OA, Jablonski MM. Natural bioadhesive biodegradable nanoparticle-based topical ophthalmic formulations for management of glaucoma. Translational vision science & technology. 2015;4(3):12–9.
Leroux JC, Allémann E, De Jaeghere F, Doelker E, Gurny R. Biodegradable nanoparticles—from sustained release formulations to improved site specific drug delivery. J Control Release. 1996;39(2–3):339–50.
Garcia-Fuentes M, Torres D, Alonso MJ. New surface-modified lipid nanoparticles as delivery vehicles for salmon calcitonin. Int J Pharm. 2005;296(1–2):122–32.
Kaur IP, Smitha R, Aggarwal D, Kapil M. Acetazolamide: future perspective in topical glaucoma therapeutics. Int J Pharm. 2002;248(1–2):1–4.
Aggarwal D, Garg A, Kaur IP. Development of a topical niosomal preparation of acetazolamide: preparation and evaluation. J Pharm Pharmacol. 2004;56(12):1509-17.
Kashiwagi K, Ito K, Haniuda H, Ohtsubo S, Takeoka S. Development of latanoprost-loaded biodegradable nanosheet as a new drug delivery system for glaucoma. Invest Ophthalmol Vis Sci. 2013;54(8):5629–37.
Nafee NA, Ismail FA, Boraie NA, Mortada LM. Mucoadhesive delivery systems. I. Evaluation of mucoadhesive polymers for buccal tablet formulation. Drug Develop Ind Pharm. 2004;30(9):985–93.
Andrews GP, Laverty TP, Jones DS. Mucoadhesive polymeric platforms for controlled drug delivery. Eur J Pharm Biopharm. 2009;71(3):505–18.
Smart JD. The basics and underlying mechanisms of mucoadhesion. Adv Drug Deliv Rev. 2005;57(11):1556–68.
Prabhu P, Kumar RN, Koland M, Harish NM, Vijayanarayan K, Dhondge G, et al. Preparation and evaluation of nano-vesicles of brimonidine tartrate as an ocular drug delivery system. J Young Pharm. 2010;2(4):356–61.
Hui-Hui Z, Qiu-Hua L, Zhi-Jun Y, Wei-San P, Shu-Fang N. Novel ophthalmic timolol meleate liposomal-hydrogel and its improved local glaucomatous therapeutic effect in vivo. Drug Del. 2011;18(7):502–10.
Li N, Zhuang C, Wang M, Sun X, Nie S, Pan W. Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. Int J Pharm. 2009;379(1):131–8.
Ishikawa M, Yoshitomi T, Zorumski CF, Izumi Y. Experimentally induced mammalian models of glaucoma. BioMed Res Int. 2015;2015.
Lu DW, Chen YH, Chang CJ, Chiang CH, Yao HY. Nitric oxide levels in the aqueous humor vary in different ocular hypertension experimental models. Kaohsiung J Med Sci. 2014;30(12):593–8.
Xu Y, Chen Z, Song J. A study of experimental carbomer glaucoma and other experimental glaucoma in rabbits. [Zhonghuayankezazhi] Chin J Ophthalmol. 2002;38(3):172–5.
Kim HG, Park JW, Park SW. Experimental chronic ocular hypertension by anterior chamber injection of 0.3% carbomer solution in the rat. Clin Exp Ophthalmol. 2013;41(4):404–12.
Liu H, Ding C. Establishment of an experimental glaucoma animal model: a comparison of microbead injection with or without hydroxypropyl methylcellulose. Exp Ther Med. 2017;14(3):1953–60.
Wong TT, Novack GD, Natarajan JV, Ho CL, Htoon HM, Venkatraman SS. Nanomedicine for glaucoma: sustained release latanoprost offers a new therapeutic option with substantial benefits over eyedrops. Drug Deliv Transl Res. 2014;4(4):303–9.
Loftsson T, Thorisdottir S, Fridriksdottir H, Stefansson E. Enalaprilat and enalapril maleate eyedrops lower intraocular pressure in rabbits. Acta Ophthalmol. 2010;88(3):337–41.
Mohsen AM, Salama A, Kassem AA. Development of acetazolamide loaded bilosomes for improved ocular delivery: preparation, characterization and in vivo evaluation. J Drug Del Sci Technol. 2020;59:101910
Manchanda S, Sahoo PK. Topical delivery of acetazolamide by encapsulating in mucoadhesive nanoparticles. Asian J Pharm Sci. 2017;12(6):550–7.
Dubey A, Prabhu P, Beladiya K, Nair N, Ghate V. Development and investigation of timolol maleate and latanoprost combination liposomes for the treatment of glaucoma. Int Res J Pharm. 2015;6(4):256–64.
Dubey A, Prabhu P. Formulation and evaluation of stimuli-sensitive hydrogels of timolol maleate and brimonidine tartrate for the treatment of glaucoma. Int J Pharm Investig. 2014;4(3):112.
Taka E, Karavasili C, Bouropoulos N, Moschakis T, Andreadis DD, Zacharis CK, Fatouros DG. Ocular co-delivery of timolol and brimonidine from a self-assembling peptide hydrogel for the treatment of glaucoma: in vitro and ex vivo evaluation. Pharmaceuticals. 2020;13(6):126.
Peng CC, Burke MT, Carbia BE, Plummer C, Chauhan A. Extended drug delivery by contact lenses for glaucoma therapy. J Control Release. 2012;162(1):152–8.
Acknowledgements
This work is issued from Pharm D thesis of Ali Bigdeli and financial support was provided by a grant from Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Funding
This work was supported by Ahvaz Jundishapur University of Medical Sciences (Grant number [N-9917].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Behzad Sharif Makhmal Zaddeh, Ali Bigdeli, Mostafa Feghhi, and Emad SoleimaniBiatiani. The first draft of the manuscript was written by Behzad Sharif Makhmal Zadeh and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (2020/02/04./ IR.AJUMS.ABHC.REC.1399.070.).”
Consent for publication
All authors are happy with this publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
bigdeli, A., Makhmalzadeh, B.S., Feghhi, M. et al. Cationic liposomes as promising vehicles for timolol/brimonidine combination ocular delivery in glaucoma: formulation development and in vitro/in vivo evaluation. Drug Deliv. and Transl. Res. 13, 1035–1047 (2023). https://doi.org/10.1007/s13346-022-01266-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01266-8