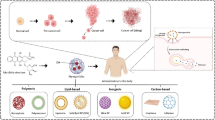
Abstract
Amphotericin B (AmB) exhibits potential antileishmanial activity, with only a little rate of recurrence. However, low bioavailability and severe nephrotoxicity are among the major shortcomings of AmB-based therapy. Various AmB nanoformulations have been developed, which to an extent, have reduced its toxicity and increased the drug efficacy. To further reduce the nonspecific tissue distribution and the cost of the treatment, the current AmB-based formulations require additional improvements. Combination of natural bioenhancers with AmB is expected to further increase its bioavailability. Therefore, we developed a nanoformulation of AmB and piperine (Pip), a plant alkaloid, known to enhance the bioavailability of various drugs, by entrapping them in guar gum, a macrophage targeting polymer. Owing to the ease of oral delivery, these nanoparticles (NPs) were coated with eudragit to make them suitable for oral administration. The formulated eudragit-coated AmB and Pip-loaded NPs (Eu-HDGG-AmB-Pip-NPs) exhibited controlled release of the loaded therapeutic agents and protected the drug from acidic pH. These NPs exhibited effective suppression of growth of both promastigotes and amastigotes of Leishmania donovani parasite under in vitro. In vivo evaluation of these NPs for therapeutic efficacy in golden hamster-L. donovani model demonstrated enhanced drug bioavailability, non-nephrotoxic nature, and potential antileishmanial activity with up to 96% inhibition of the parasite.

Graphical abstract






Similar content being viewed by others
Change history
06 March 2020
In the original version of this article, Vikas Srivastava’s last name was spelled incorrectly.
References
Kumar R, Hafner LM, Engwerda CR. Immune regulation during chronic visceral leishmaniasis. PLoS Negl Trop Dis. 2014;8(7):e2914.
Kole L, Das L, Das PK. Synergistic effect of interferon-g and mannosylated liposome incorporated doxorubicin in the therapy of experimental visceral leishmaniasis. J Infect Dis. 1999;180:811–20.
Elizabeth M, Contreras M. Chemotherapy used in the treatment of visceral leishmaniasis. CPQ Microbiol. 2019;3(1):01–14.
Soto J, Soto P. Miltefosine: oral treatment of leishmaniasis. Expert Rev Anti-Infect Ther. 2006;4:177–85.
Sundar S, Chakravarty J. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. Expert Opin Investig Drugs. 2015;24:43–59.
Paila YD, Bhaskar Saha B, Chattopadhyay A. Amphotericin B inhibits entry of Leishmania donovani into primary macrophages. Biochem Biophys Res Commun. 2010;399:429–33.
Italia JL, Yahya MM, Singh D, Ravi Kumar MN. Biodegradable nanoparticles improve oral bioavailability of amphotericin B and show reduced nephrotoxicity compared to intravenous Fungizone. Pharm Res. 2009;26(6):1324–31.
Laniado-Laborín R, Cabrales-Vargas MN. Amphotericin B: side effects and toxicity. Rev Iberoam Micol. 2009;26(4):223–7.
Zaioncz S, Khalil NM, Mainardes RM. Exploring the role of nanoparticles in amphotericin B delivery. Curr Pharm Des. 2017;23(3):509–21.
Malani PN, Depestel DD, Riddell J, Bickley S, Klein LR, Kauffman CA. Experience with community-based amphotericin B infusion therapy. Pharmacotherapy. 2005;25:690–7.
Polonio T, Efferth T. Leishmaniasis: drug resistance and natural products. Int J Mol Med. 2008;22:277–86.
Mishra BB, Kale RR, Singh RK, Tiwari VK. Alkaloids: future prospective to combat leishmaniasis. Fitoterapia. 2009;80:81–90.
Wadhwa S, Singhal S, Rawat S. Bioavailability enhancement by piperine: a review. Asian J Biomed Pharm Sci. 2014;04(36):1–8.
Randhawa KG, Kullar SJ, Rajkumar. Bioenhancer from mother nature and their applicability to modern medicines. International journal of applied and basic medicinal research. Int J App Basic Med Res. 2001;1(1):5–10.
Moretton MA, Chiappetta DA, Andrade F, Das Neves J, Ferreira D, Sarmento B, et al. Hydrolyzed galactomannan-modified nanoparticles and flower-like polymeric micelles for the active targeting of rifampicin to macrophages. J Biomed Nanotechnol. 2013;9(6):1076–87.
Bansal R, Singh A, Gandhi R, Pant AB, Kumar P, Gupta KC. Galactomannan-PEI based non-viral vectors for targeted delivery of plasmid to macrophages and hepatocytes. Eur J Pharm Biopharm. 2014;87(3):461–71.
Coviello T, Alhaique F, Dorigo A, Matricardi P, Grassi M. Two galactomannans and scleroglucan as matrices for drug delivery: preparation and release studies. Eur J Pharm Biopharm. 2007;66(2):200–9.
Nurnadiah R, Kamarun D, Li AR, Ahmad MR. Synthesis and characterization of crosslinked galactomannan nanoparticles for drug delivery application. Adv Mater Res. 2013;812:12–9.
Duncan CJ, Pugh N, Pasco DS, Ross SA. Isolation of a galactomannan that enhances macrophage activation from the edible fungus Morchella esculenta. J Agric Food Chem. 2002;50(20):5683–5.
Y-m H, Chun S-H, S-t H, Y-c K, H-d C, Lee K-w. Immune enhancing effect of a Maillard-type lysozyme-galactomannan conjugate via signaling pathways. Int J Biol Macromol. 2013;60:399–404.
Noleto GR, Mercê ALR, Iacomini M, Gorin PA, Soccol VT, Oliveira MBM. Effects of a lichen galactomannan and its vanadyl (IV) complex on peritoneal macrophages and leishmanicidal activity. Mol Cell Biochem. 2002;233(1–2):73–83.
Hosny KM. Alendronate sodium as enteric coated solid lipid nanoparticles; preparation, optimization, and in vivo evaluation to enhance its oral bioavailability. PlosOne. 2016;11(5):e0154926.
Ray L, Kumar P, Gupta KC. The activity against Ehrlich’s ascites tumors of doxorubicin contained in self assembled, cell receptor targeted nanoparticles with simultaneous oral delivery of the green tea polyphenol epigallocatechin-3-gallate. Biomaterials. 2013;34:3064–76.
Ray L, Pal MK, Ray RS. Synergism of co-delivered nanosized antioxidants displayed enhanced anticancer efficacy in human colon cancer cell lines. Bioact Mater. 2017;2:1–14.
Ashutosh, Gupta S, Ramesh, Sundar S, Goyal N. Use of Leishmania donovani field isolates expressing the luciferase reporter gene in in vitro drug screening. Antimicrob Agents Chemother. 2005;49:3776–83.
Seifert K, Croft SL. In vitro and in vivo interactions between sodium stibogluconate, miltefosine and other antileishmanial drug. Antimicrob Agents Chemother. 2006;50:73–9.
Bhatnagar S, Guru PY, Katiyar JC, Srivastava R, Mukherjee A, Akhtar MS. Exploration of antileishmanial activity in heterocycles; results of their in vivo & in vitro bioevaluations. Indian J Med Res. 1989;89:439–43.
Gershkovich P, Sivak O, Wasan EK, Magil AB, Owen D, Clement JG, et al. Biodistribution and tissue toxicity of amphotericin B in mice following multiple dose administration of a novel oral lipid-based formulation (iCo-009) J. Antimicrob Chemother. 2010;65:2610–3.
Deshpande NM, Gangrade MG, Kekarea MB, Vaidya VV. Determination of free and liposomal amphotericin B in human plasma by liquid chromatography–mass spectroscopy with solid phase extraction and protein precipitation techniques. J Chromatogr B. 2010;878:315–26.
Peters JH. The determination of creatinine and creatine in blood and urine with the photoelectric colorimeter. J. biol. Chem, 1942;146: 179-186.
Katiyar SS, Muntimadugu E, Rafeeqi AT, Domb AJ, Khan W. Co-delivery of rapamycin- and piperine-loaded polymeric nanoparticles for breast cancer treatment. Drug Deliv. 2016;23:2608–16.
Fallingborg J. Intraluminal pH of the human gastrointestinal tract. Dan Med Bull. 1999;46:183–96.
Lee KW, Everts H, Beynen AC. Essential oils in broiler nutrition. Int J Poult Sci. 2004;3:738–52.
Annamalai AR, Manavlan R. Trikatu- A bioavailability enhancer. Indian Drugs. 1989;27:595–604.
Khajuria A, Thusu N, Zutshi U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine. 2002;9:224–31.
Reen RK, Singh J. In vitro and in vivo inhibition of pulmonary cytochrome P450 activities by piperine, a major ingredient of piper species. Indian J Exp Biol. 1991;29:568–73.
Sobh MA, Moustafa FE, Ramzy RM, Deelder AM, Ghoneim MA. Schistosoma haematobium-induced glomerular disease: an experiment study in the golden hamster. Nephron. 1991;57:216–24.
Acknowledgments
KCG thanks, Indian Council of Medical Research (ICMR), New Delhi, for awarding Dr. A.S. Paintal Distinguished Scientist Chair at CSIR-IGIB, Delhi. LR thanks CSIR, New Delhi, for her senior research associate (SRA) fellowship CSIR (Pool Section) (IA-27607) at CSIR-CDRI. NG acknowledges Council of Scientific and Industrial Research Network Project, HOPE (BSC0114) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical statement
Institutional Animal Ethics Committee (IAEC) of CSIR-Central Drug Research Institute, Lucknow, reviewed and approved the animal protocol [IAEC/2012/87/Renewed 05(5/13) and IAEC/2012/87/Renewed 06(15/14)] which was adhered to National guidelines CPCSEA (Committee For the Purpose of Control and Supervision of Experiments on Animals) of Government of India. Animals were housed in plastic cages at 23 ± 2 °C, humidity 60–63%, and fed standard rodent pellet and water ad libitum and fed with standard rodent food pellet (Lipton India, Bombay).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of the article has been corrected: Vikas Srivastava’s last name was spelled incorrectly.
Rights and permissions
About this article
Cite this article
Ray, L., Karthik, R., Srivastava, V. et al. Efficient antileishmanial activity of amphotericin B and piperine entrapped in enteric coated guar gum nanoparticles. Drug Deliv. and Transl. Res. 11, 118–130 (2021). https://doi.org/10.1007/s13346-020-00712-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00712-9