Abstract
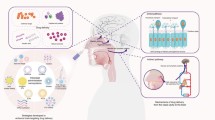
The current therapeutic strategies are not efficient in treating disorders related to the central nervous system (CNS) and have only shown partial alleviation of symptoms, as opposed to disease-modifying effects. With change in population demographics, incidence of CNS disorders, especially neurodegenerative diseases, is expected to rise dramatically. Current treatment regimens are associated with severe side-effects, especially given that most of these are chronic therapies and involve elderly population. In this review, we highlight the challenges and opportunities in delivering newer and more effective bio-therapeutic agents for the treatment of CNS disorders. Bio-therapeutics like proteins, peptides, monoclonal antibodies, growth factors, and nucleic acids are thought to have a profound effect on halting the progression of neurodegenerative disorders and also provide a unique function of restoring damaged cells. We provide a review of the nano-sized formulation-based drug delivery systems and alternate modes of delivery, like the intranasal route, to carry bio-therapeutics effectively to the brain.




Similar content being viewed by others
References
McGonigle P. Peptide therapeutics for CNS indications. Biochem Pharmacol. 2012;83(5):559–66.
Rajadhyaksha M, Boyden T, Liras J, El-Kattan A, Brodfuehrer J. Current advances in delivery of biotherapeutics across the blood–brain barrier. Curr Drug Discov Technol. 2011;8(2):87–101.
Garcel A, Martel S, Carrupt P, Doelker E, Delie F. In vitro blood brain barrier models as a screening tool for colloidal drug delivery systems and other nanosystems. Int J Biomed Nanosci Nanotechnol. 2010;1(2):133–63.
Pardridge WM. Re-engineering biopharmaceuticals for delivery to brain with molecular Trojan horses. Bioconjug Chem. 2008;19(7):1327–38.
Potschka H. Role of CNS efflux drug transporters in antiepileptic drug delivery: overcoming CNS efflux drug transport. Adv Drug Deliv Rev. 2012;64(10):943–52.
Barchet TM, Amiji MM. Challenges and opportunities in CNS delivery of therapeutics for neurodegenerative diseases. Expert Opin Drug Deliv. 2009;6(3):211–25.
Dietz GP, Bahr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol Cell Neurosci. 2004;27(2):85–131.
Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19(3):311–30.
Van RI, Cakir-Tascioglu S, Hennink WE, Storm G, Schiffelers RM, Mastrobattista E. In vivo methods to study uptake of nanoparticles into the brain. Pharm Res. 2011;28(3):456–71.
Pardridge WM. Molecular Trojan horses for blood–brain barrier drug delivery. Discov Med. 2006;6(34):139–43.
Musacchio T, Torchilin VP. Recent developments in lipid-based pharmaceutical nanocarriers. Front Biosci. 2011;16:1388–412.
Laquintana V, Trapani A, Denora N, Wang F, Gallo JM, Trapani G. New strategies to deliver anticancer drugs to brain tumors. Expert Opin Drug Deliv. 2009;6(10):1017–32.
Pardridge WM. Vector-mediated drug delivery to the brain. Adv Drug Deliv Rev. 1999;36(2–3):299–321.
Soni V, Kohli DV, Jain SK. Transferrin-conjugated liposomal system for improved delivery of 5-fluorouracil to brain. J Drug Target. 2008;16(1):73–8.
Zhang Y, Calon F, Zhu C, Boado RJ, Pardridge WM. Intravenous nonviral gene therapy causes normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism. Hum Gene Ther. 2003;14(1):1–12.
Lindqvist A, Rip J, Gaillard PJ, Bjorkman S, Hammarlund-Udenaes M. Enhanced Brain Delivery of the Opioid Peptide DAMGO in Glutathione PEGylated Liposomes: A Microdialysis Study. Mol Pharm 2012.
Zara GP, Cavalli R, Bargoni A, Fundaro A, Vighetto D, Gasco MR. Intravenous administration to rabbits of non-stealth and stealth doxorubicin-loaded solid lipid nanoparticles at increasing concentrations of stealth agent: pharmacokinetics and distribution of doxorubicin in brain and other tissues. J Drug Target. 2002;10(4):327–35.
Wong HL, Bendayan R, Rauth AM, Li Y, Wu XY. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev. 2007;59(6):491–504.
Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 2007;59(6):454–77.
Wong HL, Bendayan R, Rauth AM, Wu XY. Development of solid lipid nanoparticles containing ionically complexed chemotherapeutic drugs and chemosensitizers. J Pharm Sci. 2004;93(8):1993–2008.
Di SA, Iannitelli A, Laserra S, Sozio P. Drug delivery strategies for Alzheimer's disease treatment. Expert Opin Drug Deliv. 2011;8(5):581–603.
Sarker DK. Engineering of nanoemulsions for drug delivery. Curr Drug Deliv. 2005;2(4):297–310.
Ganta S, Deshpande D, Korde A, Amiji M. A review of multifunctional nanoemulsion systems to overcome oral and CNS drug delivery barriers. Mol Membr Biol. 2010;27(7):260–73.
Vyas TK, Shahiwala A, Amiji MM. Improved oral bioavailability and brain transport of Saquinavir upon administration in novel nanoemulsion formulations. Int J Pharm. 2008;347(1–2):93–101.
Tiwari SB, Amiji MM. A review of nanocarrier-based CNS delivery systems. Curr Drug Deliv. 2006;3(2):219–32.
Wong HL, Wu XY, Bendayan R. Nanotechnological advances for the delivery of CNS therapeutics. Adv Drug Deliv Rev. 2012;64(7):686–700.
Huwyler J, Yang J, Pardridge WM. Receptor mediated delivery of daunomycin using immunoliposomes: pharmacokinetics and tissue distribution in the rat. J Pharmacol Exp Ther. 1997;282(3):1541–6.
Liu L, Guo K, Lu J, Venkatraman SS, Luo D, Ng KC, et al. Biologically active core/shell nanoparticles self-assembled from cholesterol-terminated PEG-TAT for drug delivery across the blood–brain barrier. Biomaterials. 2008;29(10):1509–17.
Michaelis K, Hoffmann MM, Dreis S, Herbert E, Alyautdin RN, Michaelis M, et al. Covalent linkage of apolipoprotein e to albumin nanoparticles strongly enhances drug transport into the brain. J Pharmacol Exp Ther. 2006;317(3):1246–53.
Fortin D, Gendron C, Boudrias M, Garant MP. Enhanced chemotherapy delivery by intraarterial infusion and blood–brain barrier disruption in the treatment of cerebral metastasis. Cancer. 2007;109(4):751–60.
Becker I, Becker KF, Meyermann R, Hollt V. The multidrug-resistance gene MDR1 is expressed in human glial tumors. Acta Neuropathol. 1991;82(6):516–9.
Sikic BI, Fisher GA, Lum BL, Halsey J, Beketic-Oreskovic L, Chen G. Modulation and prevention of multidrug resistance by inhibitors of P-glycoprotein. Cancer Chemother Pharmacol. 1997;40(Suppl):S13–9.
Lee YJ, Maeda J, Kusuhara H, Okauchi T, Inaji M, Nagai Y, et al. In vivo evaluation of P-glycoprotein function at the blood–brain barrier in nonhuman primates using [11C]verapamil. J Pharmacol Exp Ther. 2006;316(2):647–53.
Mistry A, Stolnik S, Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm. 2009;379(1):146–57.
Illum L. Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol. 2004;56(1):3–17.
Illum L. Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol. 2004;56(1):3–17.
Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11(1):1–18.
Dhuria SV, Hanson LR, Frey II WH. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–73.
Thorne RG, Pronk GJ, Padmanabhan V, Frey WH. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–96.
Banks WA, During MJ, Niehoff ML. Brain uptake of the glucagon-like peptide-1 antagonist exendin(9–39) after intranasal administration. J Pharmacol Exp Ther. 2004;309(2):469–75.
Baker H, Spencer RF. Transneuronal transport of peroxidase-conjugated wheat germ agglutinin (WGA-HRP) from the olfactory epithelium to the brain of the adult rat. Exp Brain Res. 1986; 63(3):461–473.
Thorne RG, Pronk GJ, Padmanabhan V, Frey WH. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–96.
Alcala-Barraza SR, Lee MS, Hanson LR, McDonald AA, Frey WH, McLoon LK. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. Journal of Drug Targeting. 2010;18(3):179–90.
Clerico DM, Lanza DC, To WC. Handbook of olfaction and gustation, 2nd edn. New York: Marcel Dekker, Inc.; 2003. p. 1–16.
Gray H. Gray’s anatomy, 15th revised edition. New York: BountyBooks; 1978.
Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11(1):1–18.
Bradbury MWB, Cserr HF. Drainage of cerebral interstitial fluid and of cerebrospinal fluid into lymphatics . In: Johnston MG, editor. Experimental Biology of lymphatic circulation. Amsterdam and New York; 1985. p. 355–391.
Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5(6):514–6.
Dhanda DS, FreyWH II, Leopold D, Kompella UB. Approaches for drug deposition in the human olfactory epithelium. Drug Delivery Technology. 2005;5:64–72.
Kumar ATC, Umberkoman B, Saini KD, David GFX. Uptake of radioactivity by body fluids and tissues in rhesus monkeys after intravenous injection or intranasal spray of tritium-labelled oestradiol and progesterone. Curr Sci. 1974;43:435–9.
Sakane T, Akizuki M, Yamashita S, Sezaki H, Nadai T. Direct drug transport from the rat nasal cavity to the cerebrospinal fluid: the relation to the dissociation of the drug. J Pharm Pharmacol. 1994;46(5):378–9.
Sakane T, Akizuki M, Taki Y, Yamashita S, Sezaki H, Nadai T. Direct drug transport from the rat nasal cavity to the cerebrospinal fluid: the relation to the molecular weight of drugs. J Pharm Pharmacol. 1995;47(5):379–81.
Wang Q, Chen G, Zeng S. Pharmacokinetics of Gastrodin in rat plasma and CSF after i.n. and i.v. Int J Pharm. 2007;341(1–2):20–5.
Costantino HR, Illum L, Brandt G, Johnson PH, Quay SC. Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm. 2007;337(1–2):1–24.
Illum L. Nanoparticulate systems for nasal delivery of drugs: a real improvement over simple systems? J Pharm Sci. 2007;96(3):473–83.
Merkus FW, Verhoef JC, Schipper NG, Marttin E. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv Drug Deliv Rev. 1998;29(1–2):13–38.
Lee VHL, Yamamoto A. Penetration and enzymatic barriers to peptide and protein absorption. Advanced Drug Delivery Reviews. 1989;4(2):171–207.
Harris AS, Nilsson IM, Wagner ZG, Alkner U. Intranasal administration of peptides: nasal deposition, biological response, and absorption of desmopressin. J Pharm Sci. 1986;75(11):1085–8.
Graff CL, Pollack GM. Functional evidence for P-glycoprotein at the nose-brain barrier. Pharm Res. 2005;22(1):86–93.
Ozsoy Y. Handbook of particulate drug delivery. In: Kumar MNV, editor. California: American Scientific Publisher; 2008. p. 143.
Wang X, He H, Leng W, Tang X. Evaluation of brain-targeting for the nasal delivery of estradiol by the microdialysis method. Int J Pharm. 2006;317(1):40–6.
Smith J, Wood E, Dornish M. Effect of chitosan on epithelial cell tight junctions. Pharm Res. 2004;21(1):43–9.
Morimoto K, Katsumata H, Yabuta T, Iwanaga K, Kakemi M, Tabata Y, et al. Evaluation of gelatin microspheres for nasal and intramuscular administrations of salmon calcitonin. Eur J Pharm Sci. 2001;13(2):179–85.
Graff CL, Pollack GM. P-Glycoprotein attenuates brain uptake of substrates after nasal instillation. Pharm Res. 2003;20(8):1225–30.
Kravtzoff R, Appelqvist T, Haddouk H, Manciaux X, Cholet G, De M, I, et al. Preclinical toxicology of biovectorTM nanoparticles: part II, local tolerance, genetic toxicology and pharmacokinetics. Toxicology Letters. 1998; 95[1001]: 117.
Gao X, Wu B, Zhang Q, Chen J, Zhu J, Zhang W, et al. Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J Control Release. 2007;121(3):156–67.
Gao X, Chen J, Tao W, Zhu J, Zhang Q, Chen H, et al. UEA I-bearing nanoparticles for brain delivery following intranasal administration. Int J Pharm. 2007;340(1–2):207–15.
Migliore MM, Vyas TK, Campbell RB, Amiji MM, Waszczak BL. Brain delivery of proteins by the intranasal route of administration: a comparison of cationic liposomes versus aqueous solution formulations. J Pharm Sci. 2010;99(4):1745–61.
Hansom LR, Frey WH II, Hoekman JD, Pohl J. Lipid growth factor formulations. In: EPO, editor. 2008.
Hanson LR, Fine JM, Hoekman JD, Nguyen TM, Burns RB, Martinez PM, et al. Intranasal delivery of growth differentiation factor 5 to the central nervous system. Drug Deliv. 2012;19(3):149–54.
Werle M, Bernkop-Schnurch A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids. 2006;30(4):351–67.
Joubert J, Geldenhuys WJ, Van der Schyf CJ, Oliver DW, Kruger HG, Govender T, et al. Polycyclic cage structures as lipophilic scaffolds for neuroactive drugs. ChemMedChem. 2012;7(3):375–84.
Bodor N, Prokai L, Wu WM, Farag H, Jonalagadda S, Kawamura M, et al. A strategy for delivering peptides into the central nervous system by sequential metabolism. Science. 1992;257(5077):1698–700.
Wu J, Yoon SH, Wu WM, Bodor N. Synthesis and biological evaluations of brain-targeted chemical delivery systems of [Nva2]-TRH. J Pharm Pharmacol. 2002;54(7):945–50.
Ganta S, Amiji M. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm. 2009;6(3):928–39.
Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448(7149):39–43.
Wu D, Pardridge WM. Central nervous system pharmacologic effect in conscious rats after intravenous injection of a biotinylated vasoactive intestinal peptide analog coupled to a blood–brain barrier drug delivery system. J Pharmacol Exp Ther. 1996;279(1):77–83.
Demeule M, Currie JC, Bertrand Y, Che C, Nguyen T, Regina A, et al. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J Neurochem. 2008;106(4):1534–44.
Martini A, Muggetti L, Warchol MP. Nasal and pulmonary drug delivery systems. Expert Opinion on Therapeutic Patents. 2000;10(3):315–23.
Romeo VD, deMeireles JC, Gries WJ, Xia WJ, Sileno AP, Pimplaskar HK, et al. Optimization of systemic nasal drug delivery with pharmaceutical excipients. Adv Drug Deliv Rev. 1998;29(1–2):117–33.
Graff CL, Pollack GM. Nasal drug administration: potential for targeted central nervous system delivery. J Pharm Sci. 2005;94(6):1187–95.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2012. 46(1–3):3–26
Mackie C, Brewster M, Noppe M, Loftsson T, Lampo A. The Use of Solubilizing Excipients and Approaches to Generate Toxicology Vehicles for Contemporary Drug Pipelines. Solvent Systems and Their Selection in Pharmaceutics and Biopharmaceutics. New York: Springer; 2007. p. 221.
Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21(2):201–30.
Strasser JF, Fung LK, Eller S, Grossman SA, Saltzman WM. Distribution of 1,3-bis(2-chloroethyl)-1-nitrosourea and tracers in the rabbit brain after interstitial delivery by biodegradable polymer implants. J Pharmacol Exp Ther. 1995;275(3):1647–55.
Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–7.
Laquintana V, Trapani A, Denora N, Wang F, Gallo JM, Trapani G. New strategies to deliver anticancer drugs to brain tumors. Expert Opin Drug Deliv. 2009;6(10):1017–32.
Dahm P, Nitescu P, Appelgren L, Curelaru I. Efficacy and technical complications of long-term continuous intraspinal infusions of opioid and/or bupivacaine in refractory nonmalignant pain: a comparison between the epidural and the intrathecal approach with externalized or implanted catheters and infusion pumps. Clin J Pain. 1998;14(1):4–16.
Krewson CE, Klarman ML, Saltzman WM. Distribution of nerve growth factor following direct delivery to brain interstitium. Brain Res. 1995;680(1–2):196–206.
Ilias W, Todoroff B. Optimizing pain control through the use of implantable pumps. Med Devices (Auckl ). 2008;1:41–7.
Victorov IV, Prass K, Dirnagl U. Improved selective, simple, and contrast staining of acidophilic neurons with vanadium acid fuchsin. Brain Res Brain Res Protoc. 2000;5(2):135–9.
Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood–brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun. 2006;340(4):1085–90.
Liu XF, Fawcett JR, Hanson LR, Frey WH. The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive intranasal insulin-like growth factor-I in rats. J Stroke Cerebrovasc Dis. 2004;13(1):16–23.
Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5(6):514–6.
Huwyler J, Wu D, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci U S A. 1996;93(24):14164–9.
Alcalay RN, Giladi E, Pick CG, Gozes I. Intranasal administration of NAP, a neuroprotective peptide, decreases anxiety-like behavior in aging mice in the elevated plus maze. Neurosci Lett. 2004;361(1–3):128–31.
Dhuria SV, Hanson LR, Frey WH. Intranasal drug targeting of hypocretin-1 (orexin-A) to the central nervous system. J Pharm Sci. 2009;98(7):2501–15.
Rat D, Schmitt U, Tippmann F, Dewachter I, Theunis C, Wieczerzak E, et al. Neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) slows down Alzheimer's disease-like pathology in amyloid precursor protein-transgenic mice. FASEB J. 2011;25(9):3208–18.
Francis GJ, Martinez JA, Liu WQ, Xu K, Ayer A, Fine J, et al. Intranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabetic encephalopathy. Brain. 2008;131(Pt 12):3311–34.
Fliedner S, Schulz C, Lehnert H. Brain uptake of intranasally applied radioiodinated leptin in Wistar rats. Endocrinology. 2006;147(5):2088–94.
Ross TM, Martinez PM, Renner JC, Thorne RG, Hanson LR, Frey WH. Intranasal administration of interferon beta bypasses the blood–brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J Neuroimmunol. 2004;151(1–2):66–77.
Alcala-Barraza SR, Lee MS, Hanson LR, McDonald AA, Frey WH, McLoon LK. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. Journal of Drug Targeting. 2010;18(3):179–90.
Ma YP, Ma MM, Ge S, Guo RB, Zhang HJ, Frey WH, et al. Intranasally delivered TGF-beta1 enters brain and regulates gene expressions of its receptors in rats. Brain Res Bull. 2007;74(4):271–7.
Yu YP, Xu QQ, Zhang Q, Zhang WP, Zhang LH, Wei EQ. Intranasal recombinant human erythropoietin protects rats against focal cerebral ischemia. Neurosci Lett. 2005;387(1):5–10.
Yang JP, Liu HJ, Cheng SM, Wang ZL, Cheng X, Yu HX, et al. Direct transport of VEGF from the nasal cavity to brain. Neurosci Lett. 2009;449(2):108–11.
Draghia R, Caillaud C, Manicom R, Pavirani A, Kahn A, Poenaru L. Gene delivery into the central nervous system by nasal instillation in rats. Gene Ther. 1995;2(6):418–23.
Jerusalmi A, Morris-Downes MM, Sheahan BJ, Atkins GJ. Effect of intranasal administration of Semliki Forest virus recombinant particles expressing reporter and cytokine genes on the progression of experimental autoimmune encephalomyelitis. Mol Ther. 2003;8(6):886–94.
Oh YK, Kim JP, Hwang TS, Ko JJ, Kim JM, Yang JS, et al. Nasal absorption and biodistribution of plasmid DNA: an alternative route of DNA vaccine delivery. Vaccine. 2001;19(31):4519–25.
Kim ID, Kim SW, Lee JK. Gene knockdown in the olfactory bulb, amygdala, and hypothalamus by intranasal siRNA administration. Korean J Anat. 2009;42:285–92.
Renner DB, Frey WH, Hanson LR. Intranasal delivery of siRNA to the olfactory bulbs of mice via the olfactory nerve pathway. Neurosci Lett. 2012;513(2):193–7.
Danielyan L, Schafer R, von Ameln-Mayerhofer A, Bernhard F, Verleysdonk S, Buadze M, et al. Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson disease. Rejuvenation Res. 2011;14(1):3–16.
Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7(1):41–53.
Dhuria SV, Hanson LR, Frey WH. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–73.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, L., Yadav, S. & Amiji, M. Nanotechnology for CNS delivery of bio-therapeutic agents. Drug Deliv. and Transl. Res. 3, 336–351 (2013). https://doi.org/10.1007/s13346-013-0133-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-013-0133-3