Abstract
Aims
To investigate the changes in patient background and treatment lines between 2016–2019 and contributing factors when sodium-glucose co-transporter 2 inhibitors (SGLT2i) are newly prescribed for type 2 diabetes mellitus patients.
Methods
The subjects comprised patients who had attended outpatient clinics at the four Jikei University School of Medicine-affiliated hospitals. One-way analysis of variance was used to evaluate annual changes in patients’ characteristics. Logistic regression analysis was also used to explore factors contributing to the treatment lines.
Results
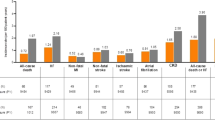
The age of the 1951 subjects [mean ± SD] was 59.1 ± 12.8 years; BMI 27.5 ± 4.9 kg/m2; HbA1c 8.15 ± 1.24%; eGFR 74.2 ± 25.3 ml/min/1.73m2. SGLT2i was the 2.86th (± 1.22) new prescription among antidiabetic drugs, and at increasingly earlier treatment lines between 2016 and 2019 (3.28 ± 1.16 to 2.59 ± 1.19; P < 0.001). The age of initial SGLT2i prescription significantly increased over time (P < 0.001). In contrast, the patients’ BMI and eGFR values decreased over time. Again, the proportions of patients with chronic kidney disease (CKD) and cardiovascular disease-heart failure disease (CVD-HF) tended to increase over time. The patients for whom SGLT2i had been prescribed in the first line were more likely to have obesity and HF (1.64 [1.15–2.34] and 1.84 [1.12–3.02], respectively).
Conclusions
SGLT2i was more likely to be newly prescribed to patients with CVD-HF and CKD, older patients, and to be prescribed in earlier treatment lines in recent years. Obesity and HF were predictor of SGLT2i prescriptions in the first line.



Similar content being viewed by others
Change history
12 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s13340-023-00685-3
References
Charvat H, Goto A, Goto M, et al. Impact of population aging on trends in diabetes prevalence: a meta-regression analysis of 160,000 Japanese adults. J Diabetes Investig. 2015;6(5):533–42.
Nakamura J, Kamiya H, Haneda M, et al. Causes of death in Japanese patients with diabetes based on the results of a survey of 45,708 cases during 2001–2010: report of the Committee on Causes of Death in Diabetes Mellitus. J Diabetes Investig. 2017;8(3):397–410.
Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77–82.
American Diabetes Association Professional Practice Committee. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S46–59.
American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S83–96.
Davies MJ, Alessio DAD, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–701.
Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int. 2020;11(3):165–223.
American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(1):S125–43.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in Type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, et al. Canagliflozin and cardiovascular and renal events in Type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetesd 2019. Diabetes Care. 2019;42(Suppl. 1):S90–102.
Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323.
Dennis JM, Henley WE, McGovern AP, et al. Time trends in prescribing of type 2 diabetes drugs, glycaemic response and risk factors: a retrospective analysis of primary care data, 2010–2017. Diabetes Obes Metab. 2019;21(7):1576–84.
Ito Y, Van Schyndle J, Nishimura T, et al. Characteristics of patients with diabetes initiating sodium glucose co-transporter-2 inhibitors (SGLT2i): real-world results from three administrative databases in Japan. Diabetes Ther. 2019;10(2):549–62.
Dave CV, Schneeweiss S, Wexler DJ, et al. Trends in clinical characteristics and prescribing preferences for SGLT2 inhibitors and GLP-1 receptor agonists, 2013–2018. Diabetes Care. 2020;43(4):921–4.
Knudsen JS, Baggese LM, Lajer M, et al. Changes in SGLT2i and GLP-1RA real-world initiator profiles following cardiovascular outcome trials: a Danish nationwide population-based study. PLoS ONE. 2020;15(3): e0229621.
Shin HJ, Schneeweiss S, Glynn RJ, et al. Trends in first-line glucose-lowering drug use in adults with type 2 diabetes in light of emerging evidence for SGLT-2i and GLP-1RA. Diabetes Care. 2021;44(8):1774–82.
Bouchi R, Sugiyama T, Goto A, et al. Retrospective nationwide study on the trends in first-line antidiabetic medication for patients with type 2 diabetes in Japan. J Diabetes Investig. 2021. https://doi.org/10.1111/jdi.13636.
Araki E, Tanizawa Y, Tanaka Y, et al. Long-term treatment with empagliflozin as add-on to oral antidiabetes therapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17(7):665–74.
Inagaki N, Kondo K, Yoshinari T, et al. Efficacy and safety of canagliflozin alone or as add-on to other oral antihyperglycemic drugs in Japanese patients with type 2 diabetes: a 52-week open-label study. J Diabetes Investig. 2015;6(2):210–8.
Kadowaki T, Haneda M, Inagaki N, et al. Efficacy and safety of empagliflozin monotherapy for 52 weeks in Japanese patients with type 2 diabetes: a randomized, double-blind, parallel-group study. Adv Ther. 2015;32(4):306–18.
Kaku K, Maegawa H, Tanizawa Y, et al. Dapagliflozin as monotherapy or combination therapy in Japanese patients with type 2 diabetes: an open-label study. Diabetes Ther. 2014;5(2):415–33.
Kashiwagi A, Kazuta K, Goto K, et al. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2015;17(3):304–8.
Kashiwagi A, Kazuta K, Yoshida S, et al. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5(4):382–91.
Seino Y, Inagaki N, Haneda M, et al. Efficacy and safety of luseogliflozin added to various oral antidiabetic drugs in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2015;6(4):443–53.
Seino Y, Sasaki T, Fukatsu A, et al. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, phase 3 study. Curr Med Res Opin. 2014;30(7):1245–55.
Tanizawa Y, Kaku K, Araki E, et al. Long-term safety and efficacy of tofogliflozin, a selective inhibitor of sodium-glucose cotransporter 2, as monotherapy or in combination with other oral antidiabetic agents in Japanese patients with type 2 diabetes mellitus: multicenter, open-label, randomized controlled trials. Expert Opin Pharmacother. 2014;15(6):749–66.
Committee on the Proper Use of SGLT2 Inhibitors. Recommendations on the Proper Use of SGLT2 Inhibitors. J Diabetes Investig. 2020;11(1):257–61.
Sumida Y, Murotani K, Saito M, et al. Effect of luseogliflozin on hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease: a prospective, single-arm trial (LEAD trial). Hepatol Res. 2019;49(1):64–71.
Xing B, Zhao Y, Dong B, et al. Effects of sodium-glucose cotransporter 2 inhibitors on non-alcoholic fatty liver disease in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. J Diabetes Investig. 2020;11(5):1238–47.
Yokote K, Terauchi Y, Nakamura I, et al. Real-world evidence for the safety of ipragliflozin in elderly Japanese patients with type 2 diabetes mellitus (STELLA-ELDER): final results of a post-marketing surveillance study. Expert Opin Pharmacother. 2016;17(15):1995–2003.
Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in Type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in Type 2 diabetes mellitus. Circulation. 2019;139(17):2022–31.
Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–9.
Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306.
Imai E, Horio M, Iseki K, et al. Prevalence of chronic kidney disease (CKD) in the Japanese general population predicted by the MDRD equation modified by a Japanese coefficient. Clin Exp Nephrol. 2007;11(2):156–63.
Ito Y, Van Schyndle J, Nishimura T, et al. Drug utilization patterns in patients with diabetes initiating sodium glucose co-transporter-2 inhibitors (SGLT2i) in Japan: a multi-database study (2014–2017). Diabetes Ther. 2019;10(6):2233–49.
Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;Suppl 1(Suppl 1):102–9.
Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. J Diabetes Investig. 2020;11(4):1020–76.
Acknowledgements
The authors would like to give special thanks to the study participants. The authors received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Rimei Nishimura has participated in speakers’ bureaus/advisory panels for Astellas, Boehringer Ingelheim, Eli Lilly, Kissei, Medtronic, Novo Nordisk, Sanofi, Takeda, Novartis, and MSD and served as subsidies or donatins for Ono, Boehringer Ingelheim, Takeda, and Taisho. The other authors haven no conflict of interest to declare.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Ethics Committee of Jikei University School of Medicine/November 12, 2018/No. 30–273 [9294]) and with the Helsinki Declaration of 1964 and later versions. This study used samples and medical information collected in the past in the course of normal medical care. Individual informed consent was not taken, but documents approved by the Ethics Committee were posted on the website to make the information available to the public, and the opportunity to refuse was provided.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Figure Sup.
Trends in patients with CKD or CVD-HF in those aged ≥ 75 years and < 75 years (2016–2019). CKD Chronic Kidney Disease, CVD-HF Cardiovascular Disease-Heart Failure. *p values for trend < 0.05 (TIF 951 KB)
About this article
Cite this article
Takahashi, H., Suganuma, Y., Ohno, T. et al. Trends in clinical characteristics and factors associated with initial prescription of SGLT2 inhibitors in Japanese patients with type 2 diabetes mellitus. Diabetol Int 13, 606–614 (2022). https://doi.org/10.1007/s13340-022-00577-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-022-00577-y