Abstract
Background
Almost 15 years after the introduction of transarterial chemoembolization (TACE) with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma (HCC) therapy, the mean peak plasma concentration (Cmax) and area under the concentration-time curve (AUC) for doxorubicin have still not been systematically reviewed or meta-analyzed.
Objective
To conduct a systematic review and meta-analysis of available data and establish a reference range for Cmax and AUC of doxorubicin DEB-TACE and TACE, as well as explore the potential influence of microspheres’ size and type on these parameters.
Methods
PubMed, EMBASE, and Web of Science were searched from August 1992 through December 2021. Studies measuring exposure parameters among HCC patients treated with doxorubicin DEB-TACE without restriction on language were included. Two independent reviewers extracted and unified data sets for pooled estimate analysis. The quality of the evidence was assessed via the Grading of Recommendations Assessment, Development and Evaluation framework. The ClinPK Statement checklist and Newcastle-Ottawa Scale (NOS) were used to determine the quality of studies.
Results
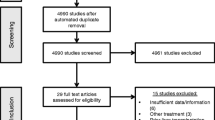
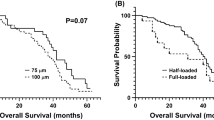
Out of 666 studies, 246 full-text were reviewed, and 8 studies entered the meta-analysis (120 patients). Cmax and AUC of doxorubicin were 7.52-fold (95% CI 7.65 to 7.42-fold; P < 0.0001) and 1.91-fold (95% CI 1.95 to 1.88-fold; P = 0.0001) lower with DEB-TACE compared to TACE. Significant reduction in pooled standardized mean difference (SMD) of Cmax and AUC was observed with DEB-TACE versus TACE in direct comparison analysis (− 2.93; 95% CI − 3.60 to − 2.26, P < 0.00001, and − 1.73 95% CI − 2.55 to − 0.91, P < 0.0001, respectively). Moreover, in DEB-TACE stratification analysis, small microspheres revealed higher Cmax, AUC and tumor response rate as well as lower complication rate.
Limitation
The heterogeneity could not be completely addressed through sensitivity and stratification analysis.
Conclusion
This meta-analysis provides exposure parameters of doxorubicin and justifies the advantage of DEB-TACE over TACE in terms of safety for patients with unresectable HCC. This study showed a marked association between the size of microsphere and exposure parameters of doxorubicin supporting the preference for small microspheres in DEB-TACE. The moderate and low quality of evidence is assigned to the Cmax and AUC, respectively.








Similar content being viewed by others
References
Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127(5 Suppl 1):S5-s16. https://doi.org/10.1053/j.gastro.2004.09.011.
Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127(5):S179–88.
Llovet J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–42. https://doi.org/10.1053/jhep.2003.50047.
Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984;226(4673):466–8. https://doi.org/10.1126/science.6093249.
King PD, Perry MC. Hepatotoxicity of chemotherapy. Oncologist. 2001;6(2):162–76. https://doi.org/10.1634/theoncologist.6-2-162.
Ohishi H, Uchida H, Yoshimura H, Ohue S, Ueda J, Katsuragi M, et al. Hepatocellular carcinoma detected by iodized oil Use of anticancer agents. Radiology. 1985;154(1):25–9. https://doi.org/10.1148/radiology.154.1.2981114.
Bhattacharya S, Novell JR, Winslet MC, Hobbs KE. Iodized oil in the treatment of hepatocellular carcinoma. Br J Surg. 1994;81(11):1563–71. https://doi.org/10.1002/bjs.1800811105.
Boulin M, Schmitt A, Delhom E, Cercueil JP, Wendremaire M, Imbs DC, et al. Improved stability of lipiodol-drug emulsion for transarterial chemoembolisation of hepatocellular carcinoma results in improved pharmacokinetic profile: proof of concept using idarubicin. Eur Radiol. 2016;26(2):601–9. https://doi.org/10.1007/s00330-015-3855-4.
de Baere T, Zhang X, Aubert B, Harry G, Lagrange C, Ropers J, et al. Quantification of tumor uptake of iodized oils and emulsions of iodized oils: experimental study. Radiology. 1996;201(3):731–5. https://doi.org/10.1148/radiology.201.3.8939223.
Deschamps F, Moine L, Isoardo T, Tselikas L, Paci A, Mir LM, et al. Parameters for stable water-in-oil lipiodol emulsion for liver trans-arterial chemo-eembolization. Cardiovasc Intervent Radiol. 2017;40(12):1927–32. https://doi.org/10.1007/s00270-017-1763-5.
Grosso M, Vignali C, Quaretti P, Nicolini A, Melchiorre F, Gallarato G, et al. Transarterial chemoembolization for hepatocellular carcinoma with drug-eluting microspheres: preliminary results from an Italian multicentre study. Cardiovasc Intervent Radiol. 2008;31(6):1141–9. https://doi.org/10.1007/s00270-008-9409-2.
Lewis AL, Taylor RR, Hall B, Gonzalez MV, Willis SL, Stratford PW. Pharmacokinetic and safety study of doxorubicin-eluting beads in a porcine model of hepatic arterial embolization. J Vasc Interv Radiol. 2006;17(8):1335–43. https://doi.org/10.1097/01.Rvi.0000228416.21560.7f.
Hulin A, Stocco J, Bouattour M. Clinical pharmacokinetics and pharmacodynamics of transarterial chemoembolization and targeted therapies in hepatocellular carcinoma. Clin Pharmacokinet. 2019;58(8):983–1014. https://doi.org/10.1007/s40262-019-00740-w.
Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46(3):474–81. https://doi.org/10.1016/j.jhep.2006.10.020.
Lilienberg E, Dubbelboer IR, Karalli A, Axelsson R, Brismar TB, Ebeling Barbier C, et al. In vivo drug delivery performance of lipiodol-based emulsion or drug-eluting beads in patients with hepatocellular carcinoma. Mol Pharm. 2017;14(2):448–58. https://doi.org/10.1021/acs.molpharmaceut.6b00886.
Kim, K.K., Pack, D.W. (2006). Microspheres for Drug Delivery. In: Ferrari, M., Lee, A.P., Lee, L.J. (eds) BioMEMS and Biomedical Nanotechnology. Springer, Boston, MA. https://doi.org/10.1007/978-0-387-25842-3_2.
Lewis AL, Dreher MR. Locoregional drug delivery using image-guided intra-arterial drug eluting bead therapy. J Control Release. 2012;161(2):338–50. https://doi.org/10.1016/j.jconrel.2012.01.018.
Dinca H, Pelage JP, Baylatry MT, Ghegediban SH, Pascale F, Manfait M. Why do small size doxorubicin-eluting microspheres induce more tissue necrosis than larger ones? A comparative study in healthy pig liver (oral communication 2206-2).
Stroup DF. Meta-analysis of Observational Studies in Epidemiology<SUBTITLE>A Proposal for Reporting</SUBTITLE>. JAMA. 2000;283(15):2008. https://doi.org/10.1001/jama.283.15.2008.
Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute; 2011. p. 1–12.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The CochraneCollaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. https://doi.org/10.1136/bmj.d5928.
Kanji S, Hayes M, Ling A, Shamseer L, Chant C, Edwards DJ, et al. Reporting guidelines for clinical pharmacokinetic studies: the ClinPK Statement. Clin Pharmacokinet. 2015;54(7):783–95. https://doi.org/10.1007/s40262-015-0236-8.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J. GRADE guidelines: introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94.
Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64(12):1311–6. https://doi.org/10.1016/j.jclinepi.2011.06.004.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. https://doi.org/10.1002/sim.1186.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557.
Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. https://doi.org/10.1186/2049-3258-72-39.
Malagari K, Kiakidis T, Pomoni M, Moschouris H, Emmanouil E, Spiridopoulos T, et al. Pharmacokinetics, safety, and efficacy of chemoembolization with doxorubicin-loaded tightly calibrated small microspheres in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2016;39(10):1379–91. https://doi.org/10.1007/s00270-016-1382-6.
van Malenstein H, Maleux G, Vandecaveye V, Heye S, Laleman W, van Pelt J, et al. A randomized phase II study of drug-eluting beads versus transarterial chemoembolization for unresectable hepatocellular carcinoma. Onkologie. 2011;34(7):368–76. https://doi.org/10.1159/000329602.
Raoul JL, Heresbach D, Bretagne JF, Ferrer DB, Duvauferrier R, Bourguet P, et al. Chemoembolization of hepatocellular carcinomas a study of the biodistribution and pharmacokinetics of doxorubicin. Cancer. 1992;70(3):585–90.
Savic LJ, Chapiro J, Funai E, Bousabarah K, Schobert IT, Isufi E, et al. Prospective study of Lipiodol distribution as an imaging marker for doxorubicin pharmacokinetics during conventional transarterial chemoembolization of liver malignancies. Eur Radiol. 2021;31(5):3002–14. https://doi.org/10.1007/s00330-020-07380-w.
Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5(9):1100–8. https://doi.org/10.1016/j.cgh.2007.04.021.
Malagari K, Pomoni M, Moschouris H, Kelekis A, Charokopakis A, Bouma E, et al. Chemoembolization of hepatocellular carcinoma with HepaSphere 30–60 μm. Safety and efficacy study. Cardiovasc Intervent Radiol. 2014;37(1):165–75. https://doi.org/10.1007/s00270-013-0777-x.
Zou JH, Zhang L, Ren ZG, Ye SL. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: a meta-analysis. J Dig Dis. 2016;17(8):510–7. https://doi.org/10.1111/1751-2980.12380.
Leung DA, Goin JE, Sickles C, Raskay BJ, Soulen MC. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12(3):321–6. https://doi.org/10.1016/s1051-0443(07)61911-3.
Choi JW, Cho HJ, Park JH, Baek SY, Chung JW, Kim DD, et al. Comparison of drug release and pharmacokinetics after transarterial chemoembolization using diverse lipiodol emulsions and drug-eluting beads. PLoS ONE. 2014;9(12): e115898. https://doi.org/10.1371/journal.pone.0115898.
Dubbelboer IR, Lilienberg E, Ahnfelt E, Sjögren E, Axén N, Lennernäs H. Treatment of intermediate stage hepatocellular carcinoma: a review of intrahepatic doxorubicin drug-delivery systems. Ther Deliv. 2014;5(4):447–66. https://doi.org/10.4155/tde.14.11.
Clark TW. Complications of hepatic chemoembolization. Semin Intervent Radiol. 2006;23(2):119–25. https://doi.org/10.1055/s-2006-941442.
Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52. https://doi.org/10.1007/s00270-009-9711-7.
Lee M, Chung JW, Lee KH, Won JY, Chun HJ, Lee HC, et al. Korean multicenter registry of transcatheter arterial chemoembolization with drug-eluting embolic agents for nodular hepatocellular carcinomas: six-month outcome analysis. J Vasc Interv Radiol. 2017;28(4):502–12. https://doi.org/10.1016/j.jvir.2016.08.017.
de Baere T, Plotkin S, Yu R, Sutter A, Wu Y, Cruise GM. An In vitro evaluation of four types of drug-eluting microspheres loaded with doxorubicin. J Vasc Interv Radiol. 2016;27(9):1425–31. https://doi.org/10.1016/j.jvir.2016.05.015.
Jordan O, Denys A, De Baere T, Boulens N, Doelker E. Comparative study of chemoembolization loadable beads: in vitro drug release and physical properties of DC bead and hepasphere loaded with doxorubicin and irinotecan. J Vasc Interv Radiol. 2010;21(7):1084–90. https://doi.org/10.1016/j.jvir.2010.02.042.
Lewis AL, Gonzalez MV, Lloyd AW, Hall B, Tang Y, Willis SL, et al. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006;17(2 Pt 1):335–42. https://doi.org/10.1097/01.Rvi.0000195323.46152.B3.
Sottani C, Poggi G, Quaretti P, Regazzi M, Montagna B, Quaquarini E, et al. Serum pharmacokinetics in patients treated with transarterial chemoembolization (TACE) using two types of epirubicin-loaded microspheres. Anticancer Res. 2012;32(5):1769–74.
Pereira PL, Plotkin S, Yu R, Sutter A, Wu Y, Sommer CM, et al. An in-vitro evaluation of three types of drug-eluting microspheres loaded with irinotecan. Anticancer Drugs. 2016;27(9):873–8. https://doi.org/10.1097/cad.0000000000000408.
Piscitelli SC, Rodvold KA, Rushing DA, Tewksbury DA. Pharmacokinetics and pharmacodynamics of doxorubicin in patients with small cell lung cancer. Clin Pharmacol Ther. 1993;53(5):555–61. https://doi.org/10.1038/clpt.1993.69.
Fuchs K, Duran R, Denys A, Bize PE, Borchard G, Jordan O. Drug-eluting embolic microspheres for local drug delivery—state of the art. J Control Release. 2017;262:127–38. https://doi.org/10.1016/j.jconrel.2017.07.016.
Faloppi L, Scartozzi M, Maccaroni E, Di Pietro PM, Berardi R, Del Prete M, et al. Evolving strategies for the treatment of hepatocellular carcinoma: from clinical-guided to molecularly-tailored therapeutic options. Cancer Treat Rev. 2011;37(3):169–77. https://doi.org/10.1016/j.ctrv.2010.08.001.
Wang Z, Zhou W, Zhang H, Qiao L. Combination of anti-angiogenesis agents and transarterial embolization: is it a promising approach for the treatment of liver cancer? Discov Med. 2015;20(108):51–5.
Namur J, Citron SJ, Sellers MT, Dupuis MH, Wassef M, Manfait M, et al. Embolization of hepatocellular carcinoma with drug-eluting beads: doxorubicin tissue concentration and distribution in patient liver explants. J Hepatol. 2011;55(6):1332–8. https://doi.org/10.1016/j.jhep.2011.03.024.
Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65(2):157–70. https://doi.org/10.1111/j.2042-7158.2012.01567.x.
Franklin RK, Marcus SA, Talaat AM, KuKanich BK, Sullivan R, Krugner-Higby LA, et al. A novel loading method for doxycycline liposomes for intracellular drug delivery: characterization of in vitro and in vivo release kinetics and efficacy in a J774A.1 cell line model of Mycobacterium smegmatis infection. Drug Metab Dispos. 2015;43(8):1236–45. https://doi.org/10.1124/dmd.115.063602.
Malagari K, Pomoni M, Moschouris H, Bouma E, Koskinas J, Stefaniotou A, et al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc Intervent Radiol. 2012;35(5):1119–28. https://doi.org/10.1007/s00270-012-0394-0.
Acknowledgments
We thank the librarian of Welch Medical Library, Johns Hopkins University, Baltimore, MD, USA, Katie Lobner, for her assistance with literature searches. Also, we thank Gorgios Sideris, MD, for participating in the initial screening.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Grant funding from RO1-CA194574.
Conflict of Interest
The authors declare that they have no conflict of interest.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Ethics approval
Not applicable.
Consent to participate and consent to publish
Not applicable.
Author contributions
MZ was involved in screening, data extraction, quality assessment, data analysis, and interpretation, designing the tables and figures, and writing of the manuscript. AK was involved in screening, data extraction, quality assessment, and designing the tables and figures; AW was involved in screening, data extraction, and quality assessment. EL was involved in the study's conceptualization, literature search, data analysis and interpretation, supervision of all the procedures, and final revision, review, and editing of the manuscript.
Rights and permissions
About this article
Cite this article
Zarisfi, M., Kasaeian, A., Wen, A. et al. Systematic Review and Pharmacokinetic Meta-analysis of Doxorubicin Exposure in Transcatheter Arterial Chemoembolization and Doxorubicin-Eluted Beads Chemoembolization for Treatment of Unresectable Hepatocellular Carcinoma. Eur J Drug Metab Pharmacokinet 47, 449–466 (2022). https://doi.org/10.1007/s13318-022-00762-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00762-z