Abstract
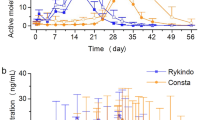
The maximum plasma concentration (C max) and the area under the plasma concentration–time curve (AUC) are commonly used to establish bioequivalence between two formulations of the same oral medication. Similarly, these pharmacokinetic parameters have also been used to establish bioequivalence between two sites of administration for the same injectable formulation. However, these conventional methods of establishing bioequivalence are of limited use when comparing modified-release formulations of a drug, particularly those with rates of absorption that are amenable to change with the site of injection. Inherent differences in the rate of absorption can result in clinically significant differences in early exposure and drug response. Here, we propose the use of the partial AUC (pAUC) as a measure of early exposure to aid in the assessment of bioequivalence between the gluteal and the deltoid site of administration for long-acting injectable antipsychotics.
Similar content being viewed by others
References
Masand PS, Roca M, Turner MS, Kane JM. Partial adherence to antipsychotic medication impacts the course of illness in patients with schizophrenia. Prim Care Companion J Clin Psychiatry. 2009;11(4):147–54.
Waddell L, Taylor M. Attitudes of patients and mental health staff to antipsychotic long-acting injections: systematic review. Br J Psychiatry. 2009;195(52):S43–50.
Zyprexa Relprevv (olanzapine) for extended release injectable suspension. Highlights of prescribing information. Eli Lilly and Company. Revised September 2015. http://pi.lilly.com/us/zyprexa_relprevv.pdf. Accessed 26 Feb 2016.
Abilify Maintena™ (aripiprazole for prolonged release injectable suspension) product monograph. Otsuka Pharmaceutical Co., Ltd. Tokyo, 101-8535 Japan. Date of Preparation: September 15, 2015. Accessed 26 Feb 2016.
Invega Sustenna® (paliperidone palmitate prolonged-release injectable suspension) product monograph. Janssen Inc. Toronto, Ontario. Date of Revision: November 25, 2015. Accessed 26 Feb 2016.
Invega Trinza™ (paliperidone palmitate extended-release injectable suspension, for intramuscular use). Highlights of prescribing information. Janssen Pharmaceuticals, Inc. Revised January 2016. https://www.janssenmd.com/pdf/invega-trinza/INVEGA-TRINZA_PI.pdf. Accessed 26 Feb 2016.
Risperdal Consta® (risperidone powder for injectable prolonged-release suspension) product monograph. Janssen Inc. Toronto, Ontario. Date of Revision: May 4, 2015. Accessed 26 Feb 2016.
Federal Food, Drug, and Cosmetic Act, 21 CFR §320.23(b). http://www.ecfr.gov/cgi-bin/text-idx?SID=82ba8cca2311b5aa75f015e1b8d373c9&mc=true&node=se21.5.320_123&rgn=div8.
Thyssen A, Rusch S, Herben V, Quiroz J, Mannaert E. Risperidone long-acting injection: pharmacokinetics following administration in deltoid versus gluteal muscle in schizophrenic patients. J Clin Pharmacol. 2010;50(9):1011–21.
Federal Food, Drug, and Cosmetic Act, 21 CFR §320.22(d)(2). http://www.ecfr.gov/cgi-bin/text-idx?SID=82ba8cca2311b5aa75f015e1b8d373c9&mc=true&node=se21.5.320_122&rgn=div8.
U.S. Food and Drug Administration. Statistical Approaches to Establishing Bioequivalence. Population and Individual Bioequivalence Working Group. Prepared January 31, 2001. http://www.fda.gov/downloads/Drugs/…/Guidances/ucm070244.pdf. Accessed 26 Feb 2016.
The European Agency for the Evaluation of Medicinal Products. Note for Guidance on the Investigation of Bioavailability and Bioequivalence. Revised July 26, 2001. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003008.pdf. Accessed 26 Feb 2016.
U.S. Food and Drug Administration. Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA. Division of Bioequvialence in the Office of Generic Drugs. Prepared December 2013. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm377465.pdf. Accessed 28 Apr 2016.
European Medicines Agency. Guideline on the Investigation of Bioequivalence. Revised January 20, 2010. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf. Accessed 28 Apr 2016.
Chen ML, Lesko L, Williams RL. Measures of exposure versus measures of rate and extent of absorption. Clin Pharmacokinet. 2001;40(8):565–72.
Lacey LF, Keene ON, Duquesnoy D, Bye A. Evaluation of different indirect measures of rate of drug absorption in comparative pharmacokinetic studies. J Pharm Sci. 1994;83(2):212–5.
Endrenyi L, Fritsch S, Yan W. C max/AUC is a clearer measure than C max for absorption rates in investigations of bioequivalence. Int J Clin Pharmacol Ther Toxicol. 1991;29(10):394–9.
Nightingale SL, Morrison JC. Generic drugs and the prescribing physician. JAMA. 1987;258(9):1200–4.
Chen ML. An alternative approach for assessment of rate of absorption in bioequivalence studies. Pharm Res. 1992;9(11):1380–5.
Bois FY, Tozer TN, Hauck WW, Chen ML, Patnaik R, Williams RL. Bioequivalence: performance of several measures of rate of absorption. Pharm Res. 1994;11(7):966–74.
Rostami-Hodjegan A, Jackson PR, Tucker GT. Sensitivity of indirect metrics for assessing “rate” in bioequivalence studies—moving the “goalposts” or changing the “game”. J Pharm Sci. 1994;83(11):1554–7.
Rosenbaum SE, Rhodes CT, Bon C. Area under the curve estimation in bioequivalence studies. Drug Dev Ind Pharm. 1990;16(1):157–63.
Endrenyi L, Tothfalusi L. Do regulatory bioequivalence requirements adequately reflect the therapeutic equivalence of modified-release drug products? J Pharm Pharm Sci. 2010;13(1):107–13.
Tozer TN, Bois FY, Hauck WW, Chen ML, Williams RL. Absorption rate vs. exposure: which is more useful for bioequivalence testing? Pharm Res. 1996;13(3):453–6.
Kondra PM, Endrenyi L, Tothfalusi L. The need for additional metrics to assess therapeutic equivalence of some multiphasic modified-release products. Clin Ther. 2011;33(9):1214–9.
Chen ML, Davit B, Lionberger R, Wahba Z, Ahn HY, Yu LX. Using partial area for evaluation of bioavailability and bioequivalence. Pharm Res. 2011;28(8):1939–47.
U.S. Food and Drug Administration. Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations. Revised March 2003. http://www.fda.gov/ohrms/dockets/ac/03/briefing/3995B1_07_GFI-BioAvail-BioEquiv.pdf. Accessed 28 Apr 2016.
U.S. Food and Drug Administration. Guidance on Zolpidem. Finalized October 2011. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm175029.pdf. Accessed 28 Apr 2016.
Yin J, Collier AC, Barr AM, Honer WG, Procyshyn RM. Paliperidone palmitate long-acting injectable given intramuscularly in the deltoid versus the gluteal muscle: are they therapeutically equivalent? J Clin Psychopharmacol. 2015;35(4):447–9.
Evans EF, Proctor JD, Fratkin MJ, Velandia J, Wasserman AJ. Blood flow in muscle groups and drug absorption. Clin Pharmacol Ther. 1975;17(1):44–7.
Procyshyn RM, Banasch JL, Barr AM, Honer WG. Breakthrough symptoms after switching paliperidone palmitate long acting injectable from the gluteal to the deltoid site of administration. J Psychiatry Neurosci. 2016;41(3):E56–7.
Elliott ES, Purvis TL, Nelson LA, Sommi RW. Inconsistency in risperidone long-acting injection steady-state plasma levels when switching from deltoid to gluteal administration. J Clin Pharmacol. 2010;50(6):721–4.
U.S. Food and Drug Administration. Draft Guidance on Risperidone. Revised May 2015. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm201272.pdf. Accessed 26 Feb 2016.
Zha J, Endrenyi L. Variation of the peak concentration following single and repeated drug administrations in investigations of bioavailability and bioequivalence. J Biopharm Stat. 1997;7(1):191–204.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Procyshyn has been a member of the following advisory boards in the past three years: Janssen, Lundbeck, and Otsuka; a member of the following speaker’s bureaus in the past three years: Janssen, Lundbeck, and Otsuka; and received grants from the Canadian Institutes of Health Research. Dr. Gershkovich has received consultancy fees from iCo Therapeutics, and have services rendered contract with TesoRx. Dr. Honer has received consulting fees or sat on paid advisory boards for: MDH Consulting, In Silico (no honorarium), Novartis, Eli Lilly, Roche, Otsuka, and Lundbeck; received honoraria from Rush University, the Fraser, Vancouver Coastal and the Providence Health Authorities; and received grants from the Canadian Institutes of Health Research. Dr. Barr has received grants from Bristol-Myers Squibb and the Canadian Institutes of Health Research. L.H.N. Lee and C. Choi declare that they have no conflicts of interest.
Sources of Funding
Canadian Institutes of Health Research, the BC Mental Health and Substance Use Services, and Jack Bell Chair in Schizophrenia.
Rights and permissions
About this article
Cite this article
Lee, L.H.N., Choi, C., Gershkovich, P. et al. Proposing the Use of Partial AUC as an Adjunctive Measure in Establishing Bioequivalence Between Deltoid and Gluteal Administration of Long-Acting Injectable Antipsychotics. Eur J Drug Metab Pharmacokinet 41, 659–664 (2016). https://doi.org/10.1007/s13318-016-0348-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-016-0348-z