Abstract
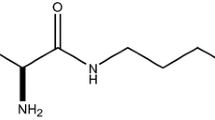
(R)-2-Amino-1,3′,3′-trimethyl-7′-(pyrimidin-5-yl)-3′,4′-dihydro-2′H-spiro[imidazole-4,1′-naphthalen]-5(1H)-one (GNE-892) is an orally administered inhibitor of β-secretase 1 (β-site amyloid precursor protein cleaving enzyme 1, BACE1) that was developed as an intervention therapy against Alzheimer’s disease. A clinical microdosing strategy was being considered for de-risking the potential pharmacokinetic liabilities of GNE-892. We tested whether dose-proportionality was observed in cynomolgus monkey as proof-of-concept for a human microdosing study. With cryopreserved monkey hepatocytes, concentration-dependency for substrate turnover and the relative contribution of P450- versus AO-mediated metabolism were observed. Characterization of the kinetics of these metabolic pathways demonstrated differences in the affinities of P450 and AO for GNE-892, which supported the metabolic profiles that had been obtained. To test if this metabolic shift occurred in vivo, mass balance studies in monkeys were conducted at doses of 0.085 and 15 mg/kg. Plasma exposure of GNE-892 following oral administration was more than 20-fold greater than dose proportional at the high-dose. P-gp-mediated efflux was unable to explain the discrepancy. The profiles of metabolites in circulation and excreta were indicative that oxidative metabolism limited the exposure to unchanged GNE-892 at the low dose. Further, the in vivo data supported the concentration-dependent metabolic shift between P450 and AO. In conclusion, microdosing of GNE-892 was not predictive of pharmacokinetics at a more pharmacologically relevant dose due to saturable absorption and metabolism. Therefore, it is important to consider ADME liabilities and their potential concentration-dependency when deciding upon a clinical microdosing strategy.






Similar content being viewed by others
Abbreviations
- ADME:
-
Absorption, distribution, metabolism, excretion
- AO:
-
Aldehyde oxidase
- AUC:
-
Area under concentration–time curve
- BACE:
-
β-Site amyloid precursor protein cleaving enzyme
- BDC:
-
Bile-duct cannulated
- C max :
-
Maximum plasma concentration
- MDCK:
-
Madin–Darby canine kidney cell line
- MDR1:
-
Human multidrug resistance gene
- P450:
-
Cytochrome P450
- P-gp:
-
P-glycoprotein
- PK:
-
Pharmacokinetics
References
Balani SK, Nagaraja NV, Qian MG, Costa AO, Daniels JS, Yang H, Shimoga PR, Wu JT, Gan LS, Lee FW, Miwa GT (2006) Evaluation of microdosing to assess pharmacokinetic linearity in rats using liquid chromatography–tandem mass spectrometry. Drug Metab Dispos 34(3):384–388. doi:10.1124/dmd.105.007195
Beedham C, Bruce SE, Critchley DJ, al-Tayib Y, Rance DJ (1987) Species variation in hepatic aldehyde oxidase activity. Eur J Drug Metab Pharmacokinet 12(4):307–310
Garattini E, Fratelli M, Terao M (2008) Mammalian aldehyde oxidases: genetics, evolution and biochemistry. Cell Mol Life Sci 65(7–8):1019–1048. doi:10.1007/s00018-007-7398-y
Garner RC (2010) Practical experience of using human microdosing with AMS analysis to obtain early human drug metabolism and PK data. Bioanalysis 2(3):429–440. doi:10.4155/bio.10.6
Hardy J, Allsop D (1991) Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci 12(10):383–388
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256(5054):184–185
Hop CE, Wang Z, Chen Q, Kwei G (1998) Plasma-pooling methods to increase throughput for in vivo pharmacokinetic screening. J Pharm Sci 87(7):901–903. doi:10.1021/js970486q
Hunt KW, Cook AW, Watts RJ, Clark CT, Vigers G, Smith D, Metcalf AT, Gunawardana IW, Burkard M, Cox AA, Geck Do MK, Dutcher D, Thomas AA, Rana S, Kallan NC, DeLisle RK, Rizzi JP, Regal K, Sammond D, Groneberg R, Siu M, Purkey H, Lyssikatos JP, Marlow A, Liu X, Tang TP (2013) Spirocyclic beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibitors: from hit to lowering of cerebrospinal fluid (CSF) amyloid beta in a higher species. J Med Chem 56(8):3379–3403. doi:10.1021/jm4002154
Hutzler JM, Obach RS, Dalvie D, Zientek MA (2013) Strategies for a comprehensive understanding of metabolism by aldehyde oxidase. Expert Opin Drug Metab Toxicol 9(2):153–168. doi:10.1517/17425255.2013.738668
Lappin G (2010) Microdosing: current and the future. Bioanalysis 2(3):509–517. doi:10.4155/bio.09.177
Liu X, Wong H, Scearce-Levie K, Watts RJ, Coraggio M, Shin YG, Peng K, Wildsmith KR, Atwal JK, Mango J, Schauer SP, Regal K, Hunt KW, Thomas AA, Siu M, Lyssikatos J, Deshmukh G, Hop CE (2013) Mechanistic pharmacokinetic-pharmacodynamic modeling of BACE1 inhibition in monkeys: development of a predictive model for amyloid precursor protein processing. Drug Metab Dispos 41(7):1319–1328. doi:10.1124/dmd.112.050864
McLean MA, Tam CYJ, Baratta MT, Holliman CL, Ings RM, Galluppi GR (2007) Accelerating drug development: methodology to support first-in-man pharmacokinetic studies by the use of drug candidate microdosing. Drug Dev Res 68(1):14–22. doi:10.1002/Ddr.20160
Ni J, Ouyang H, Aiello M, Seto C, Borbridge L, Sakuma T, Ellis R, Welty D, Acheampong A (2008) Microdosing assessment to evaluate pharmacokinetics and drug metabolism in rats using liquid chromatography–tandem mass spectrometry. Pharm Res 25(7):1572–1582. doi:10.1007/s11095-008-9555-x
Powis G, Ames MM, Kovach JS (1979) Metabolic conversion of indicine N-oxide to indicine in rabbits and humans. Cancer Res 39(9):3564–3570
Pryde DC, Dalvie D, Hu Q, Jones P, Obach RS, Tran TD (2010) Aldehyde oxidase: an enzyme of emerging importance in drug discovery. J Med Chem 53(24):8441–8460. doi:10.1021/jm100888d
Rowland M (2012) Microdosing: a critical assessment of human data. J Pharm Sci 101(11):4067–4074. doi:10.1002/jps.23290
Sandhu P, Vogel JS, Rose MJ, Ubick EA, Brunner JE, Wallace MA, Adelsberger JK, Baker MP, Henderson PT, Pearson PG, Baillie TA (2004) Evaluation of microdosing strategies for studies in preclinical drug development: demonstration of linear pharmacokinetics in dogs of a nucleoside analog over a 50-fold dose range. Drug Metab Dispos 32(11):1254–1259. doi:10.1124/dmd.104.000422
Shou M, Lin Y, Lu P, Tang C, Mei Q, Cui D, Tang W, Ngui JS, Lin CC, Singh R, Wong BK, Yergey JA, Lin JH, Pearson PG, Baillie TA, Rodrigues AD, Rushmore TH (2001) Enzyme kinetics of cytochrome P450-mediated reactions. Curr Drug Metab 2(1):17–36
Strelevitz TJ, Orozco CC, Obach RS (2012) Hydralazine as a selective probe inactivator of aldehyde oxidase in human hepatocytes: estimation of the contribution of aldehyde oxidase to metabolic clearance. Drug Metab Dispos 40(7):1441–1448. doi:10.1124/dmd.112.045195
Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M (1999) Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286(5440):735–741
Witherow LE, Houston JB (1999) Sigmoidal kinetics of CYP3A substrates: an approach for scaling dextromethorphan metabolism in hepatic microsomes and isolated hepatocytes to predict in vivo clearance in rat. J Pharmacol Exp Ther 290(1):58–65
Yamazaki A, Kumagai Y, Yamane N, Tozuka Z, Sugiyama Y, Fujita T, Yokota S, Maeda M (2010) Microdose study of a P-glycoprotein substrate, fexofenadine, using a non-radioisotope-labelled drug and LC/MS/MS. J clin pharm ther 35:169–175
Acknowledgments
We thank Matthew Durk, Jialin Mao, and Sophie Mukadam for helpful discussions and Ronitte Libedinsky for editorial contributions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takahashi, R., Ma, S., Yue, Q. et al. Dose-dependent exposure and metabolism of GNE-892, a β-secretase inhibitor, in monkeys: contributions by P450, AO, and P-gp. Eur J Drug Metab Pharmacokinet 40, 171–185 (2015). https://doi.org/10.1007/s13318-014-0198-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-014-0198-5