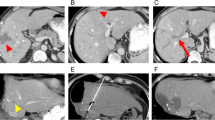
Abstract
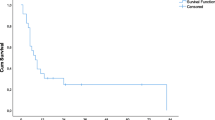
Standard treatment of early recurrence of colorectal liver metastases (CLM) after liver resection (LR) is chemotherapy followed by loco-regional therapy. We reviewed the outcome of a different strategy (“test-of-time” approach): upfront percutaneous ablation without chemotherapy. Twenty-six consecutive patients with early solitary liver-only recurrence amenable to both resection and ablation (< 30 mm, distant from vessels) undergone “test-of-time” approach were analyzed. Early recurrence had a median size of 17 mm and occurred after a median interval from LR of 4 months. Primary efficacy rate of ablation was 100%. Five patients are alive and disease-free after a mean follow-up of 46 months. Five patients had local-only recurrence; all had repeat treatment (LR = 4; Ablation = 1) without chemotherapy. Local recurrence risk was associated with incomplete ablation of 1-cm thick peritumoral margin. The remaining 16 patients had non-local recurrence, 13 early after ablation. Overall, six (23%) patients had ablation as unique treatment and 13 (50%) avoided or postponed chemotherapy (mean chemotherapy-free interval 33.5 months). Ablation without chemotherapy of early liver-only recurrence is a reliable “test-of-time” approach. It minimized the invasiveness of treatment with good effectiveness and high salvageability in case of local failure, avoided worthless surgery, and saved chemotherapy for further disease progression.




Similar content being viewed by others
References
Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J (1994) Factors influencing the natural history of colorectal liver metastases. Lancet (London, England) 343(8910):1405–1410
Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM (2006) Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 244(2):254–259
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aguilar EA, Bardelli A, Benson A, Bodoky G, Ciardiello F, D’Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Arnold D et al (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27(8):1386–1422. https://doi.org/10.1093/annonc/mdw235
Creasy JM, Sadot E, Koerkamp BG, Chou JF, Gonen M, Kemeny NE, Balachandran VP, Kingham TP, DeMatteo RP, Allen PJ, Blumgart LH, Jarnagin WR, D’Angelica MI (2018) Actual 10-year survival after hepatic resection of colorectal liver metastases: what factors preclude cure? Surgery 163(6):1238–1244. https://doi.org/10.1016/j.surg.2018.01.004
Viganò L, Ferrero A, Tesoriere RL, Capussotti L (2008) Liver surgery for colorectal metastases: results after 10 years of follow-up. Long-term survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol 15(9):2458–2464. https://doi.org/10.1245/s10434-008-9935-9
Viganò L, Pedicini V, Comito T, Carnaghi C, Costa G, Poretti D, Franzese C, Personeni N, Del Fabbro D, Rimassa L, Scorsetti M, Santoro A, Solbiati L, Torzilli G (2018) Aggressive and multidisciplinary local approach to iterative recurrences of colorectal liver metastases. World J Surg 42(8):2651–2659
Wicherts DA, de Haas RJ, Salloum C, Andreani P, Pascal G, Sotirov D, Adam R, Castaing D, Azoulay D (2013) Repeat hepatectomy for recurrent colorectal metastases. Br J Surg 100(6):808–818
Fukami Y, Kaneoka Y, Maeda A, Takayama Y, Onoe S (2017) Postoperative complications following aggressive repeat hepatectomy for colorectal liver metastasis have adverse oncological outcomes. Surg Today 47(1):99–107
Viganò L, Capussotti L, Lapointe R, Barroso E, Hubert C, Giuliante F, Ijzermans JN, Mirza DF, Elias D, Adam R (2014) Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6025 patients. Ann Surg Oncol 21(4):1276–1286
Pathak S, Jones R, Tang JM, Parmar C, Fenwick S, Malik H, Poston G (2011) Ablative therapies for colorectal liver metastases: a systematic review. Colorectal Dis 13(9):e252–e265
Gillams A, Goldberg N, Ahmed M, Bale R, Breen D, Callstrom M, Chen MH, Choi BI, de Baere T, Dupuy D, Gangi A, Gervais D, Helmberger T, Jung EM, Lee F, Lencioni R, Liang P, Livraghi T, Lu D, Meloni F, Solbiati L (2015) Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, the interventional oncology Sans Frontières meeting 2013. Eur Radiol 25(12):3438–3454
van Amerongen MJ, Jenniskens S, van den Boezem PB, Fütterer JJ, de Wilt J (2017) Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases—a meta-analysis. HPB (Oxford) 19(9):749–756
Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN (2012) Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 265(3):958–968
Van Tilborg AA, Meijerink MR, Sietses C, Van Waesberghe JH, Mackintosh MO, Meijer S, Van Kuijk C, Van Den Tol P (2011) Long-term results of radiofrequency ablation for unresectable colorectal liver metastases: a potentially curative intervention. Br J Radiol 84(1002):556–565
Elias D, De Baere T, Smayra T, Ouellet JF, Roche A, Lasser P (2002) Percutaneous radiofrequency thermoablation as an alternative to surgery for treatment of liver tumour recurrence after hepatectomy. Br J Surg 89(6):752–756
Sofocleous CT, Petre EN, Gonen M, Brown KT, Solomon SB, Covey AM, Alago W, Brody LA, Thornton RH, D’Angelica M, Fong Y, Kemeny NE (2011) CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J Vasc Interv Radiol 22(6):755–761
Dupré A, Jones RP, Diaz-Nieto R, Fenwick SW, Poston GJ, Malik HZ (2017) Curative-intent treatment of recurrent colorectal liver metastases: a comparison between ablation and resection. Eur J Surg Oncol 43(10):1901–1907
Valls C, Ramos E, Leiva D, Ruiz S, Martinez L, Rafecas A (2015) Safety and efficacy of ultrasound-guided radiofrequency ablation of recurrent colorectal cancer liver metastases after hepatectomy. Scand J Surg 104(3):169–175
Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Garrido-Laguna I, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Freedman-Cass DA et al (2018) NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw 16(4):359–369
Otto G, Düber C, Hoppe-Lotichius M, König J, Heise M, Pitton MB (2010) Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann Surg 251(5):796–803
Meijerink MR, Puijk RS, van Tilborg A, Henningsen KH, Fernandez LG, Neyt M, Heymans J, Frankema JS, de Jong KP, Richel DJ, Prevoo W, Vlayen J (2018) Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis. Cardiovasc Intervent Radiol 41(8):1189–1204
Gurusamy K, Corrigan N, Croft J, Twiddy M, Morris S, Woodward N, Bandula S, Hochhauser D, Napp V, Pullan A, Jakowiw N, Prasad R, Damink SO, van Laarhoven C, de Wilt J, Brown J, Davidson BR (2018) Liver resection surgery versus thermal ablation for colorectal LiVer MetAstases (LAVA): study protocol for a randomised controlled trial. Trials 19(1):105
Puijk RS, Ruarus AH, Vroomen L, van Tilborg A, Scheffer HJ, Nielsen K, de Jong MC, de Vries J, Zonderhuis BM, Eker HH, Kazemier G, Verheul H, van der Meijs BB, van Dam L, Sorgedrager N, Coupé V, van den Tol P, Meijerink MR, COLLISION Trial Group (2018) Colorectal liver metastases: surgery versus thermal ablation (COLLISION)—a phase III single-blind prospective randomized controlled trial. BMC Cancer 18(1):821
Livraghi T, Solbiati L, Meloni F, Ierace T, Goldberg SN, Gazelle GS (2003) Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test-of-time approach.” Cancer 97(12):3027–3035
Imai K, Allard MA, Benitez CC, Vibert E, Cunha AS, Cherqui D, Castaing D, Bismuth H, Baba H, Adam R (2016) Early recurrence after hepatectomy for colorectal liver metastases: what optimal definition and what predictive factors? Oncologist 21(7):887–894
Solbiati M, Muglia R, Goldberg SN, Ierace T, Rotilio A, Passera KM, Marre I, Solbiati L (2019) A novel software platform for volumetric assessment of ablation completeness. Int J Hyperthermia 36(1):337–343
Torzilli G, Viganò L, Gatti A, Costa G, Cimino M, Procopio F, Donadon M, Del Fabbro D (2017) Twelve-year experience of “radical but conservative” liver surgery for colorectal metastases: impact on surgical practice and oncologic efficacy. HPB (Oxford) 19(9):775–784
Viganò L, Costa G, Cimino MM, Procopio F, Donadon M, Del Fabbro D, Belghiti J, Kokudo N, Makuuchi M, Vauthey JN, Torzilli G (2018) R1 resection for colorectal liver metastases: a survey questioning surgeons about its incidence, clinical impact, and management. J Gastrointest Surg 22(10):1752–1763
Vigano L, Darwish SS, Rimassa L, Cimino M, Carnaghi C, Donadon M, Procopio F, Personeni N, Del Fabbro D, Santoro A, Torzilli G (2018) Progression of colorectal liver metastases from the end of chemotherapy to resection: a new contraindication to surgery? Ann Surg Oncol 25(6):1676–1685
Viganò L, Torzilli G, Troisi R, Aldrighetti L, Ferrero A, Majno P, Toso C, Figueras J, Cherqui D, Adam R, Kokudo N, Hasegawa K, Guglielmi A, Krawczyk M, Giuliante F, Hilal MA, Costa-Maia J, Pinna AD, Cescon M, De Santibanes E, CLISCO Group et al (2019) Minor hepatectomies: focusing a blurred picture: analysis of the outcome of 4471 open resections in patients without cirrhosis. Ann Surg 270(5):842–851
Viganò L, Torzilli G, Aldrighetti L, Ferrero A, Troisi R, Figueras J, Cherqui D, Adam R, Kokudo N, Hasegawa K, Guglielmi A, Majno P, Toso C, Krawczyk M, Abu Hilal M, Pinna AD, Cescon M, Giuliante F, De Santibanes E, Costa-Maia J, CLISCO Group et al (2020) Stratification of major hepatectomies according to their outcome: analysis of 2212 consecutive open resections in patients without cirrhosis. Ann Surg 272(5):827–833
Pang YY (2002) The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333-39. HPB (Oxford) 4(2):99–100
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, Chen MH, Choi BI, de Baère T, Dodd GD 3rd, Dupuy DE, Gervais DA, Gianfelice D, Gillams AR, Lee FT Jr, Leen E, Lencioni R, Littrup PJ, Livraghi T, Lu DS, Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe et al (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology 273(1):241–260
Takahashi H, Berber E (2020) Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg Nutr 9(1):49–58
Mulier S, Ruers T, Jamart J, Michel L, Marchal G, Ni Y (2008) Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? An update. Dig Surg 25(6):445–460
Dupré A, Lefranc A, Buc E, Delpero JR, Quenet F, Passot G, Evrard S, Rivoire M (2013) Use of bioresorbable membranes to reduce abdominal and perihepatic adhesions in 2-stage hepatectomy of liver metastases from colorectal cancer: results of a prospective, randomized controlled phase II trial. Ann Surg 258(1):30–36
Kron P, Linecker M, Jones RP, Toogood GJ, Clavien PA, Lodge J (2019) Ablation or resection for colorectal liver metastases? A systematic review of the literature. Front Oncol 9:1052
van Duijnhoven FH, Jansen MC, Junggeburt JM, van Hillegersberg R, Rijken AM, van Coevorden F, van der Sijp JR, van Gulik TM, Slooter GD, Klaase JM, Putter H, Tollenaar RA (2006) Factors influencing the local failure rate of radiofrequency ablation of colorectal liver metastases. Ann Surg Oncol 13(5):651–658
Huo YR, Eslick GD (2015) Microwave ablation compared to radiofrequency ablation for hepatic lesions: a meta-analysis. J Vasc Interv Radiol 26(8):1139-1146.e2
Di Martino M, Rompianesi G, Mora-Guzmán I, Martín-Pérez E, Montalti R, Troisi RI (2020) Systematic review and meta-analysis of local ablative therapies for resectable colorectal liver metastases. Eur J Surg Oncol 46(5):772–781
Shady W, Petre EN, Do KG, Gonen M, Yarmohammadi H, Brown KT, Kemeny NE, D’Angelica M, Kingham PT, Solomon SB, Sofocleous CT (2018) Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol 29(2):268-275.e1
Torzilli G, Del Fabbro D, Palmisano A, Marconi M, Makuuchi M, Montorsi M (2007) Salvage hepatic resection after incomplete interstitial therapy for primary and secondary liver tumours. Br J Surg 94(2):208–213
Brouquet A, Vauthey JN, Badgwell BD, Loyer EM, Kaur H, Curley SA, Abdalla EK (2011) Hepatectomy for recurrent colorectal liver metastases after radiofrequency ablation. Br J Surg 98(7):1003–1009
Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S (2008) Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology (Baltimore, MD) 47(1):82–89
Kaye EA, Cornelis FH, Petre EN, Tyagi N, Shady W, Shi W, Zhang Z, Solomon SB, Sofocleous CT, Durack JC (2019) Volumetric 3D assessment of ablation zones after thermal ablation of colorectal liver metastases to improve prediction of local tumor progression. Eur Radiol 29(5):2698–2705
Shady W, Petre EN, Vakiani E, Ziv E, Gonen M, Brown KT, Kemeny NE, Solomon SB, Solit DB, Sofocleous CT (2017) Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget 8(39):66117–66127
Calandri M, Yamashita S, Gazzera C, Fonio P, Veltri A, Bustreo S, Sheth RA, Yevich SM, Vauthey JN, Odisio BC (2018) Ablation of colorectal liver metastasis: interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur Radiol 28(7):2727–2734
Odisio BC, Yamashita S, Huang SY, Harmoush S, Kopetz SE, Ahrar K, Chun YS, Conrad C, Aloia TA, Gupta S, Hicks ME, Vauthey JN (2017) Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br J Surg 104(6):760–768
Acknowledgements
The authors thank Marco Solbiati (RAW-Endosight, Milan, Italy) for his contribution in imaging analysis with the Ablation-fitTM software.
Funding
The authors have no funding sources to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Research involving human participants and/or animals
The present study complies with the guidelines for human studies. The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Informed consent
The Institutional Review Board of our hospital approved this retrospective study and the requirement of informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vigano, L., Galvanin, J., Poretti, D. et al. Percutaneous ablation of post-surgical solitary early recurrence of colorectal liver metastases is an effective “test-of-time” approach. Updates Surg 73, 1349–1358 (2021). https://doi.org/10.1007/s13304-021-01047-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-021-01047-x