Abstract
Introduction
Emerging evidence showed that adipocytes are important regulators in controlling insulin resistance in type 2 diabetes mellitus (T2DM). So far, compounds isolated from natural plants have been widely studied for their roles in alleviating T2DM-associated complications. This work evaluated the actions of astragaloside IV (AS-IV) on insulin resistance and inflammatory biomarker expression in adipocytes and explored the potential mechanisms.
Methods
Glucose consumption of the adipocytes was determined by a glucose assay kit; the mRNA expression levels of glucose transporter type 4 (GLUT-4), interleukin-6 (IL-6), TNF-α and C1q tumor necrosis factor-related protein 3 (CTRP3) were measured by quantitative real-time PCR (qRT-PCR); the protein levels were determined by western blot assay and enzyme-linked immunosorbent assay.
Results
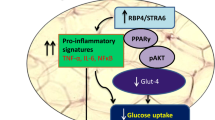
AS-IV concentration-dependently increased glucose consumption in the insulin resistance adipocytes. Further qRT-PCR results showed that AS-IV concentration-dependently reduced adipocyte IL-6 and TNF-α expression. Moreover, GLUT-4 expression in adipocytes was also significantly upregulated by AS-IV. Furthermore, we found that AS-IV concentration-dependently increased CTRP3 expression in adipocytes. CTRP3 silence decreased glucose consumption, upregulated IL-6 and TNF-α expression and downregulated GLUT-4 mRNA expression in 200 µM AS-IV-treated adipocytes. Moreover, AS-IV treatment enhanced the activity of phosphoinositide 3-kinase (PI3K)/AKT signaling in adipocytes, which was markedly attenuated by CTRP3 silencing. Importantly, inhibition of PI3K/AKT signaling also attenuated AS-IV induced an increase in glucose consumption and GLUT-4 expression and a decrease in IL-6 and TNF-α expression of adipocytes.
Conclusions
Collectively, our data indicated that AS-IV attenuated insulin resistance and inflammation in adipocytes via targeting CTRP3/PI3K/Akt signaling.
Similar content being viewed by others
AS-IV concentration-dependently increased glucose consumption in the insulin resistance adipocytes |
AS-IV concentration-dependently reduced adipocyte interleukin-6 (IL-6) and tumor-necrosis factor-alpha (TNF-α) expression |
AS-IV concentration-dependently increased C1q tumor necrosis factor-related protein 3 (CTRP3) expression in adipocytes |
AS-IV treatment enhanced the activity of PI3K/AKT signaling in adipocytes, which was markedly attenuated by CTRP3 silencing |
Inhibition of PI3K/AKT signaling also attenuated AS-IV-induced increase in glucose consumption and GLUT-4 expression and decrease in IL-6 and TNF-α expression of adipocytes |
Introduction
Type 2 diabetes mellitus (T2DM) is a frequently occurring metabolic disease, and T2MD prevalence is steadily increasing in the world [1, 2]. In T2DM, patients usually develop impaired utilization of insulin in metabolic tissues and organs, which can cause high blood glucose levels and associated complications including renal failure, obesity, cardiovascular dysfunction and even cancers [3, 4]. Insulin resistance is defined by impaired efficacy in utilizing insulin by effector organs. Insulin resistance can cause declining glucose uptake and elimination in various organs/tissues such as adipose tissues, which has been regarded as an important contributor to T2DM pathophysiology [5, 6]. However, the underlying molecular mechanisms remain elusive.
Recent studies have extensively investigated the role of adipose tissue in T2DM pathophysiology by using different interventions by attenuating insulin resistance in the adipocytes. Bailey et al. demonstrated that monocarboxylate transporter 1 could enhance adipocyte proliferation and insulin sensitivity [7]. Gao et al. revealed that bovine α-lactalbumin-derived peptides attenuate tumor necrosis factor-α (TNF-α)-induced inflammation and insulin resistance of adipocytes by inhibiting NF-κB and JNK signaling [8]. Shimizu et al. showed that resolvin E3 could regulate phosphoinositide 3-kinase (PI3K)/AKT signaling and alleviate insulin resistance by high fat induction in adipocytes [9]. Recently, studies also showed that miR-27a regulated insulin resistance in 3T3-L1 cells by targeting peroxisome proliferator-activated receptor (PPAR) gamma [10]. Therefore, to further identify novel targets/compounds to mediate insulin resistance in adipocytes may represent a good strategy for combating this disease.
Astragaloside IV (AS-IV) is a type of saponin and can be extracted from Astragalus membranaceus. AS-IV exhibited various biological actions such as anti-information, anti-oxidation and immune enhancement [11]. AS-IV also exerted protective actions against T2DM-associated complications. However, actions of AS-IV in modulating inflammation and insulin resistance in adipocytes have not been fully documented. Thus, we examined the actions of AS-IV in insulin resistance and inflammatory markers of adipocytes. Furthermore, we also examined the interaction between AS-IV and other downstream mediators including C1q tumor necrosis factor-related protein 3 (CTRP3) and PI3K/Akt signaling pathway. This work may provide promising insights into protective actions of AS-IV against T2DM. It is based on in vitro studies using cell lines and does not contain any studies with human participants or animals performed by any of the authors.
Methods
Culture of Adipocytes and Establishment of Insulin-Resistant Adipocytes
The preadipocyte line 3T3-L1 cells were from ATCC (Manassas). Preadipocytes were cultured in DMEM (Sigma) supplemented with 10% fetal calf serum (Sigma) at 5% CO2, 37 °C atmosphere. After preadipocytes reached confluence, preadipocytes were processed for adipocyte differentiation by incubating with following reagents for 5 days: DMEM/F12/glutamate-medium containing 3-isobutyl-methyl-xanthine (0.5 mM), insulin (1 μM), panthothenate (20 μM), ascorbate (200 μM), biotin (1 μM), pedersenfetuin (300 mg/l) and transferrin (2 μg/ml). After that, adipocytes were stimulated with DMEM/F12/glutamate medium containing insulin (1 nM) till they differentiated into adipocytes (differentiated type).
To establish adipocytes with insulin resistance, differentiated adipocytes were stimulated with Krebs-Ringer phosphate (KRP) buffer supplemented with palmitic acid (1 mM), glucose (10 nM) and 1% bovine serum albumin (BSA) overnight For control 3T3-L1 cells, they were incubated in KPR buffer with 1% BSA overnight. Then, 3T3-L1 cells were washed with KRP buffer containing 1% BSA and incubated for 1 h at 37 °C; 3T3-L1 cells were rinsed in KRP buffer containing 1% BSA and were maintained in this buffer for downstream assays.
Treatment of AS-IV, siRNA and Wortmannin
For the AS-IV (Sigma) treatment, the adipocytes were treated with different concentrations (10, 50, 100 and 200 µM) of AS-IV for 24 h, and after treatment, adipocytes were processed for downstream assays. The siRNA that targets CTRP3 (5′- TGGATTTCGUGGUUACCAATT-3′) was synthesized by RiboBio (Guangzhou, China), and siRNA with the scrambled sequence (5′-GTTCAGTTCAGGTTAGTATCT-3′) was used as control siRNA. 3T3-L1 cells were transfected with scrambled siRNA or CTRP3 siRNA using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's protocol, and 3T3-L1 cells were processed for downstream assays after the transfections for 24 h. For the wortmannin (Sigma), 3T3-L1 cells were pre-treated with wortmannin (100 nM) for 1 h before collecting for downstream assays.
Glucose Consumption
3T3-L1 glucose consumption was assessed in culture medium using a glucose assay kit (Beyotime, Beijing, China). Briefly, 3T3-L1 cells were allowed to grow till 70–80% confluence. After treatments with AS-IV, CTRP3 siRNA or wortmannin mentioned above, glucose level in supernatant was determined with a glucose assay kit. The amount of glucose consumption was the total glucose content in the medium minus the remnant glucose.
Quantitative Real-Time PCR (qRT-PCR)
To measure mRNA expression, qRT-PCR assay was carried out. RNA isolation was carried out with Trizol reagent (Abmbion) following the manufacturer’s guidelines. RNA quality/concentrations were assessed with a Nanodrop 2000 system (ThermoFisher). cDNA was obtained from 2 μg RNA with First Strand cDNA Synthesis kit (ThermoFisher). Analyses of qPCR were carried out in an ABI7900 system (Applied Biosystem) with SYBR Select Master Mix (Applied Biosystems, Life Technologies, Foster City, CA, USA). Glucose transporter type 4 (GLUT-4), interleukin-6 (IL-6), TNF-α and C1q tumor necrosis factor-related protein 3 (CTRP3) expression was calculated with 2−ΔΔCt formula. GAPDH was used as reference gene. The primer sequences were as follow: GLUT-4, forward, 5′-CCCCGCTGGAATGAGGTTTTTGAGGTGAT-3′, reverse, 5′-CAGACAGGGGCCGAAGATTGGGAGACAGT-3′; IL-6, forward, 5′-AGTTGCCTTCTTGGGACTGA-3′, reverse, 5′-CAGAATTGCCATTGCACAAC-3′; TNF-α, 5′-ACGGCATGGATCTCAAAGAC-3′, reverse, 5′-CGGCAGAGACCACCTTGAACT-3′; CTRP3, forward, 5′-GAGTCTCCACAAACCGGAGG-3′, reverse, 5′-TCACCTTTGTCGCCCTTCTC-3′; GAPDH, forward, 5′-CAACGGATTTGGTCGTATTGG-3′, reverse, 5′-GCAACAATATCCACTTTACCAGAGTTAA-3′.
Enzyme-Linked Immunosorbent Assay (ELISA)
After treatments with AS-IV, CTRP3 siRNA or wortmannin mentioned above, supernatant from the culture medium of 3T3-L1 cells was used to measure IL-6 and TNF-α using corresponding ELISA kits (R&D Systems, USA) as per the protocol of the manufacturer.
Western Blot Assay
The adipocytes, after treatments with AS-IV, CTRP3 siRNA or wortmannin, were used for protein extraction with RIPA buffer containing the protease inhibitor cocktail (Sigma). The protein concentrations of the extracted samples were determined by bicinchoninic acid protein assay kit (Thermal Fisher Scientific). Equal amounts of proteins (30 µg) were resolved on 10% sodium dodecyl sulfate-polyacrylamide electrophoresis gels and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore). Then, PVDF membranes were blocked with 6% skimmed milk for 1.5 h followed with incubation by antibodies against phosphorylated PI3K (p-PI3K; 1:1000, Cell Signaling Technology), PI3K (1:1000, Cell Signaling Technology), phosphorylated AKT (p-AKT; 1:1000, Cell Signaling Technology), AKT (1:1000, Cell Signaling Technology), CTRP3 and β-actin (1:3000, Cell Signaling Technology). Membranes were probed with corresponding horseradish peroxidase-conjugated secondary antibodies (1:2000; Cell Signaling Technology). The bands were detected by the Enhanced Chemiluminescence Kit (Thermo Fisher Scientific). Protein levels were analyzed using ImageJ software using β-actin for normalization.
Statistical Analysis
All statistical analysis was assessed with GraphPad Prism 6.0 (GraphPadPrism Software, La Jolla). Results are expressed as the mean ± standard deviation, and P < 0.05 was considered statistically significant. Statistical analysis between groups was carried out by one-way analysis of variance followed by Turkey’s post hoc analysis.
Results
Actions of AS-IV on Insulin Resistance and Inflammatory Response in Adipocytes
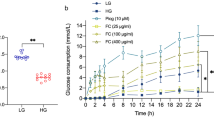
First, we assessed glucose consumption of adipocytes stimulated with escalating concentrations of AS-IV. The glucose consumption in adipocytes with insulin resistance without AS-IV treatment was lower than that of normal adipocytes. Moreover, AS-IV at 10 μM failed to increase glucose consumption detected in insulin-resistant adipocytes; AS-IV from 50 to 200 μM concentration-dependently increased glucose consumption in insulin-resistant adipocytes compared to 0 μM AS-IV group (Fig. 1A). Inflammatory-associated mediators such as IL-6 and TNF-α in adipocytes were assessed after AS-IV treatments. AS-IV at 10 μM did not affect IL-6 and TNF-α mRNA expression compared to 0 μM AS-IV group (Fig. 1B, C); AS-IV from 50 to 200 μM elevated IL-6 and TNF-α mRNA expression in adipocytes compared to 0 μM group (Fig. 1B, C). Consistently, IL-6 and TNF-α protein levels in adipocytes were not affected by 10 μM AS-IV compared to 0 μM group (Fig. 1D, E); IL-6 and TNF-α protein levels in adipocytes were concentration-dependently reduced by AS-IV from 50 to 200 μM compared to 0 μM AS-IV group (Fig. 1D, E). Moreover, GLUT-4 mRNA expression was not increased by 10 μM AS-IV compared to 0 μM AS-IV group (Fig. 1F), while AS-IV from 50 to 200 μM concentration-dependently enhanced GLUT-4 mRNA expression in adipocytes (Fig. 1G).
Actions of AS-IV on insulin resistance and inflammatory response in adipocytes. A Actions of AS-IV in glucose consumption in adipocytes. B Actions of AS-IV on IL-6 mRNA expression in adipocytes. C Actions of AS-IV on TNF-α mRNA expression in adipocytes. D Actions of AS-IV on IL-6 protein levels in adipocytes. E Actions of AS-IV on TNF-α protein level in adipocytes. F Actions of AS-IV on GLUT-4 mRNA expression in adipocytes. N = 3. *P < 0.05 and **P < 0.01
Effects of AS-IV on the CTRP3 Expression in Adipocytes
Furthermore, we assessed whether AS-IV could modulate CTRP3 expression in adipocytes. AS-IV at 10 μM did not elevate CTRP3 mRNA expression in adipocytes compared to 0 μM AS-IV group (Fig. 2A); 50–200 μM AS-IV could dramatically elevate the CTRP3 mRNA level of adipocytes compared to 0 μM AS-IV group (Fig. 2A). Consistently, we found that AS-IV from 50 to 200 μM but not 10 μM increased CTRP3 protein expression in adipocytes compared to 0 μM group (Fig. 2B). To study CTRP3 functions in adipocytes, loss-of-function study was undertaken. CTRP3 mRNA expression was reduced in adipocytes after CRTP3 siRNA transfection compared to si-NC group (Fig. 2C). Consistently, reduced CTRP3 protein level in adipocytes was also detected after CTRP3 siRNA transfection (Fig. 2D).
Actions of AS-IV on the CTRP3 expression in adipocytes. A Effects of AS-IV on CTRP3 mRNA expression in adipocytes. B Actions of AS-IV on CTRP3 protein level in adipocytes. C Effects of CTRP3 siRNA or si-NC transfection on CTRP3 mRNA expression in adipocytes. D Effects of CTRP3 siRNA or si-NC transfection on CTRP3 protein level in adipocytes. N = 3. **P < 0.01 and ***P < 0.001
Effects of CTRP3 Silencing on Insulin Resistance and Inflammatory Response in Adipocytes Treated with AS-IV
For the glucose consumption, 200 μM AS-IV increased adipocytes glucose consumption, which was attenuated by CTRP3 silencing (Fig. 3A). IL-6 and TNF-α mRNA expression was downregulated in 200 μM AS-IV group compared to control group, while 200 μM AS-IV + CTRP3 siRNA group exhibited higher IL-6 and TNF-α mRNA expression in adipocytes than that in 200 μM AS-IV group (Fig. 3B, C). Consistently, CTRP3 siRNA treatment attenuated 200 μM AS-IV-induced elevation in IL-6 and TNF-α protein levels of adipocytes (Fig. 3D, E). Moreover, 200 μM AS-IV enhanced adipocyte GLUT-4 expression in adipocytes, which was attenuated by CTRP3 silencing (Fig. 3F).
Effects of CTRP3 silencing on the insulin resistance and inflammatory response in adipocytes treated with AS-IV. A Effects of CTRP3 silencing on glucose consumption in AS-IV stimulated AS-IV are shown. B Effects of CTRP3 silencing on IL-6 mRNA expression in AS-IV stimulated AS-IV are shown. C Effects of CTRP3 silencing on TNF-α mRNA expression in AS-IV stimulated AS-IV are shown. D Effects of CTRP3 silencing on IL-6 protein levels in AS-IV stimulated AS-IV are shown. E Effects of CTRP3 silencing on TNF-α protein level in AS-IV stimulated AS-IV are shown. F Effects of CTRP3 silencing on GLUT-4 mRNA expression in AS-IV stimulated AS-IV are shown. *P < 0.05, **P < 0.01 and ***P < 0.001
Effects of CTRP3 Silencing on the PI3K/AKT Signaling in Adipocytes Treated with AS-IV
The actions of CTRP3 silencing on PI3K/ATK signaling in adipocytes treated with AS-IV were further assessed with western blot assay. Total PI3K and total AKT protein levels of adipocytes were not affected by AS-IV treatment or CTRP3 siRNA (Fig. 4); 200 μM AS-IV treatment enhanced p-PI3K and p-AKT protein expression in adipocytes, which partially reversed CTRP3 silencing (Fig. 4).
Effects of Wortmannin on the Insulin Resistance and Inflammatory Response in Adipocytes Treated with AS-IV
For glucose consumption, 200 μM AS-IV increased adipocyte glucose consumption, which was attenuated by wortmannin (Fig. 5A). IL-6 and TNF-α mRNA expression was downregulated in 200 μM AS-IV group compared to control group, while 200 μM AS-IV + wortmannin NA group exhibited higher IL-6 and TNF-α mRNA expression in adipocytes than that in 200 μM AS-IV group (Fig. 5B, C). Consistently, wortmannin treatment attenuated 200 μM AS-IV-induced elevation in IL-6 and TNF-α protein levels of adipocytes (Fig. 5D, E). Moreover, 200 μM AS-IV enhanced adipocyte GLUT-4 expression in adipocytes, which was attenuated by wortmannin (Fig. 5F).
Actions of wortmannin on insulin resistance and inflammatory response in adipocytes treated with AS-IV. A Effects of wortmannin on glucose consumption in AS-IV stimulated AS-IV are shown. B Effects of wortmannin on IL-6 mRNA expression in AS-IV stimulated AS-IV are shown. C Effects of wortmannin on TNF-α mRNA expression in AS-IV stimulated AS-IV are shown. D Effects of wortmannin on IL-6 protein levels in AS-IV stimulated AS-IV are shown. E Effects of wortmannin on TNF-α protein level in AS-IV stimulated AS-IV are shown. F Effects of wortmannin on GLUT-4 mRNA expression in AS-IV stimulated AS-IV are shown. *P < 0.05 and **P < 0.01
Discussion
Impaired insulin utilization is a key feature in T2DM, and emerging evidence showed that adipocytes are important regulators in controlling insulin resistance in T2DM [12, 13]. So far, compounds isolated from natural plants have been widely studied for their roles in alleviating T2DM-associated complications [14]. This work evaluated the effects of AS-IV on insulin resistance and inflammatory biomarker expression in adipocytes and explored the potential mechanisms. AS-IV concentration-dependently increased the glucose consumption in the insulin-resistant adipocytes. Further qRT-PCR results showed that AS-IV concentration-dependently reduced adipocyte IL-6 and TNF-α expression. Moreover, GLUT-4 mRNA expression in adipocytes was also significantly upregulated by AS-IV. Furthermore, we found that AS-IV concentration-dependently increased CTRP3 expression in adipocytes. CTRP3 silence decreased glucose consumption, upregulated IL-6 and TNF-α expression and downregulated GLUT-4 mRNA expression in 200 µM AS-IV-treated adipocytes. Moreover, AS-IV treatment enhanced the activity of PI3K/AKT signaling in adipocytes, which was markedly attenuated by CTRP3 silencing. Importantly, inhibition of PI3K/AKT signaling also attenuated AS-IV-induced increase in glucose consumption and GLUT-4 expression and decrease in adipocyte IL-6 and TNF-α expression. Collectively, this work indicated that AS-IV attenuated insulin resistance and inflammation in adipocytes via targeting CTRP3/PI3K/Akt signaling.
The actions of AS-IV on glucose uptake/insulin resistance have been reported in various studies. Xu et al. showed AS-IV at 30 µg/ml could alleviate insulin resistance induced by high glucose in preadipocytes [15]. AS-IV at 12.5–50 µM was effective in facilitating glucose transport in C2C12 myotubes, and the proposed mechanism was related to insulin receptor substrate-1/AKT signaling [16]. In the HepG2 cells, AS-IV at 25.6, 51.2 and 102.4 μM increased glucose uptake [17]. Wang et al. showed that AS-IV could exert protective effects on diabetic cardiomyopathy via promoting metabolism of myocardial lipid [18]; AS-IV could also attenuate T2DM-associated liver injury by promoting AMP-activated protein kinase/mammalian target of rapamycin-mediated autophagy [19]. Recently, Gong et al. showed that AS-IV exhibited a hypoglycemic effect in T2DM mice by modulating PI3K/AKT pathway [20]. Consistently, our results revealed that AS-IV ranging from 50 to 200 µM effectively increased glucose consumption in the adipocytes, and this effect may be associated with increase GLUT-4 expression. Moreover, we also showed that AS-IV could reduce inflammation under diabetic conditions. AS-IV could reduce high-glucose-stimulated inflammatory reactions in HUVECs by inhibiting c-Jun N-terminal kinase pathway [21]. Gui et al. demonstrated AS-IV ameliorated injury in kidney tissues via regulating inflammatory response in diabetic rats [22]. Shi et al. showed that AS-IV inhibited inflammation through TLR4/MyD88/NF-κB pathway in acute myocardial infarction [23]. Moreover, Feng et al. showed that AS-IV ameliorated kidney injury by affecting NLRP3 inflammasome-associated inflammation in db/db mice [24]. In a consistent manner, we found that AS-IV could concentration-dependently suppress IL-6 and TNF-α expression in adipocytes, suggesting that AS-IV could repress inflammation in adipocytes.
CTRP3 belongs to the CTRPs family, which can produce an anti-diabetic effect similar to that of adiponectin [25]. Studies from the meta-analysis results showed that circulating CTPR3 level was negatively correlated with T2DM status [26]. A clinical trial also showed that plasma CTRP3 concentrations was inversely associated with insulin resistance in Chinese patients with obesity and T2DM [27]. Yao et al. demonstrated that CTRP3 was a potential diagnostic factor for diabetic retinopathy and could attenuate high glucose-induced VCAM-1 [28]. In the adipocytes, CTRP3 negatively regulated lipid metabolism during adipocyte differentiation [29], and CTPR3 could improve adipocyte insulin sensitivity by attenuating inflammation and inhibiting insulin signaling activity [30]. In our results, we showed that AS-IV concentration-dependently upregulated CTRP3 expression in adipocytes, and silence of CTRP3-attenuated AS-IV induced an increase in glucose consumption and GLUT-4 expression and a decrease in IL-6 and TNF-α expression in adipocytes, suggesting that AS-IV exerted protective effects via upregulating CTRP3 in adipocytes.
PI3K/Akt signaling pathway involves various biological functions. Studies have demonstrated that AS-IV and CTRP3 both can interact with PI3K/Akt signaling pathway. For example, AS-IV could alleviate subarachnoid hemorrhage-induced brain injury through PI3K/Akt signaling [31]. AS-IV exerted a hypoglycemic effect via regulating PI3K/Akt pathway [20]. On the other hand, CRTP3 could alleviate apoptosis of mesenchymal stem cells caused by serum deprivation and hypoxia via PI3K/Akt pathway [32]. Chen et al. demonstrated that CTRP3 alleviated endothelial dysfunction and inflammation caused by Ox-LDL in mouse aortic endothelial cells via PI3K/Akt pathway [33]. Recent studies revealed that CTRP3 overexpression exerted neuroprotective effects against sevoflurane-induced cognitive impairment via PI3K/AKT pathway in rats [34]. Our results showed that AS-IV activated PI3K/AKT signaling in adipocytes, which was attenuated by CTRP3 silence. Moreover, inhibition of PI3K/AKT signaling also attenuated an AS-IV-induced increase in glucose consumption and GLUT-4 expression and decrease in IL-6 and TNF-α expression in adipocytes. The above evidence indicated that AS-IV exerted its biological function in adipocytes possibly via regulating CTRP3 and PI3K/AKT signaling.
There are several limitations to this study. First, the present study only focused on the in vitro cellular experiments, while further in vivo studies should be performed to determine the effects of AS-IV on insulin resistance and inflammatory response of adipocytes. Second, the weakness of the study was a lack of comparison of effects of AS-IV with other antidiabetic agents having strict similarity in the actions, such as PPAR gamma antagonists. Third, this study only examined the PI3K/AKT signaling pathway, and further studies should consider exploring other related signaling pathways to fully decipher the AS-IV-mediated actions in adipocytes.
Conclusions
In conclusion, this study showed that AS-IV functioned to attenuate insulin resistance and inflammatory response in adipocytes, and mechanistic studies indicated that AS-IV exerted these effects via modulating CTRP3 and PI3K/AKT signaling in adipocytes. As metabolically unhealthy obesity includes whole-body insulin resistance, hepatic steatosis and subclinical inflammation, clinically, AS-IV may be more effective in managing metabolically unhealthy obesity than metabolically healthy obesity.
References
Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet (London, England). 2005;365:1333–46.
Taylor R. Type 2 diabetes: etiology and reversibility. Diabetes Care. 2013;36:1047–55.
Gloyn AL, Drucker DJ. Precision medicine in the management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018;6:891–900.
Tinajero MG, Malik VS. An update on the epidemiology of type 2 diabetes: a global perspective. Endocrinol Metab Clin N Am. 2021;50:337–55.
Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6.
Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98:2133–223.
Bailey T, Nieto A, McDonald P. Inhibition of the monocarboxylate transporter 1 (MCT1) promotes 3T3-L1 adipocyte proliferation and enhances insulin sensitivity. Int J Mol Sci. 2022;23:1901.
Gao J, Guo K, Du M, Mao X. Bovine α-lactalbumin-derived peptides attenuate TNF-α-induced insulin resistance and inflammation in 3T3-L1 adipocytes through inhibiting JNK and NF-κB signaling. Food Funct. 2022;13:2323–35.
Shimizu T, Saito T, Aoki-Saito H, Okada S, Ikeda H, Nakakura T, et al. Resolvin E3 ameliorates high-fat diet-induced insulin resistance via the phosphatidylinositol-3-kinase/Akt signaling pathway in adipocytes. FASEB J. 2022;36: e22188.
Zhuang Y, Li M. MiRNA-27a mediates insulin resistance in 3T3-L1 cells through the PPARγ. Mol Cell Biochem. 2022;477:1107–12.
Zhang J, Wu C, Gao L, Du G, Qin X. Astragaloside IV derived from Astragalus membranaceus: a research review on the pharmacological effects. Adv Pharmacol (San Diego, Calif). 2020;87:89–112.
Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21:6275.
Solis-Herrera C, Triplitt C, Cersosimo E, DeFronzo RA, et al. Pathogenesis of type 2 diabetes mellitus. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al., editors. Endotext. South Dartmouth: MDText.com Inc.; 2000.
Blahova J, Martiniakova M, Babikova M, Kovacova V, Mondockova V, Omelka R. Pharmaceutical Drugs and natural therapeutic products for the treatment of type 2 diabetes mellitus. Pharmaceuticals (Basel, Switzerland). 2021;14:806.
Xu ME, Xiao SZ, Sun YH, Ou-Yang Y, Zheng XX. Effects of astragaloside IV on pathogenesis of metabolic syndrome in vitro. Acta Pharmacol Sin. 2006;27:229–36.
Zhu R, Zheng J, Chen L, Gu B, Huang S. Astragaloside IV facilitates glucose transport in C2C12 myotubes through the IRS1/AKT pathway and suppresses the palmitate-induced activation of the IKK/IκBα pathway. Int J Mol Med. 2016;37:1697–705.
Zhou X, Wang LL, Tang WJ, Tang B. Astragaloside IV inhibits protein tyrosine phosphatase 1B and improves insulin resistance in insulin-resistant HepG2 cells and triglyceride accumulation in oleic acid (OA)-treated HepG2 cells. J Ethnopharmacol. 2021;268: 113556.
Wang Z, Zhu Y, Zhang Y, Zhang J, Ji T, Li W, et al. Protective effects of AS-IV on diabetic cardiomyopathy by improving myocardial lipid metabolism in rat models of T2DM. Biomed Pharmacother Biomed Pharmacother. 2020;127:110081.
Zhu Y, Su Y, Zhang J, Zhang Y, Li Y, Han Y, et al. Astragaloside IV alleviates liver injury in type 2 diabetes due to promotion of AMPK/mTOR-mediated autophagy. Mol Med Rep. 2021;23:437.
Gong P, Xiao X, Wang S, Shi F, Liu N, Chen X, et al. Hypoglycemic effect of astragaloside IV via modulating gut microbiota and regulating AMPK/SIRT1 and PI3K/AKT pathway. J Ethnopharmacol. 2021;281: 114558.
You L, Fang Z, Shen G, Wang Q, He Y, Ye S, et al. Astragaloside IV prevents high glucose-induced cell apoptosis and inflammatory reactions through inhibition of the JNK pathway in human umbilical vein endothelial cells. Mol Med Rep. 2019;19:1603–12.
Gui D, Huang J, Guo Y, Chen J, Chen Y, Xiao W, et al. Astragaloside IV ameliorates renal injury in streptozotocin-induced diabetic rats through inhibiting NF-κB-mediated inflammatory genes expression. Cytokine. 2013;61:970–7.
Shi H, Zhou P, Gao G, Liu PP, Wang SS, Song R, et al. Astragaloside IV prevents acute myocardial infarction by inhibiting the TLR4/MyD88/NF-κB signaling pathway. J Food Biochem. 2021;45: e13757.
Feng H, Zhu X, Tang Y, Fu S, Kong B, Liu X. Astragaloside IV ameliorates diabetic nephropathy in db/db mice by inhibiting NLRP3 inflammasome-mediated inflammation. Int J Mol Med. 2021;48:164.
Yan Z, Cao X, Wang C, Liu S, Li Y, Lu G, et al. C1q/tumor necrosis factor-related protein-3 improves microvascular endothelial function in diabetes through the AMPK/eNOS/NO· signaling pathway. Biochem Pharmacol. 2022;195: 114745.
Moradi N, Najafi M, Sharma T, Fallah S, Koushki M, Peterson JM, et al. Circulating levels of CTRP3 in patients with type 2 diabetes mellitus compared to controls: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2020;169: 108453.
Qu H, Deng M, Wang H, Wei H, Liu F, Wu J, et al. Plasma CTRP-3 concentrations in Chinese patients with obesity and type II diabetes negatively correlate with insulin resistance. J Clin Lipidol. 2015;9:289–94.
Yan Z, Zhao J, Gan L, Zhang Y, Guo R, Cao X, et al. CTRP3 is a novel biomarker for diabetic retinopathy and inhibits HGHL-induced VCAM-1 expression in an AMPK-dependent manner. PLoS ONE. 2017;12: e0178253.
Nishimoto H, Yamamoto A, Furukawa S, Wakisaka S, Maeda T. C1q/TNF-related protein 3 expression and effects on adipocyte differentiation of 3T3-L1 cells. Cell Biol Int. 2017;41:197–203.
Li X, Jiang L, Yang M, Wu YW, Sun JZ, Sun SX. CTRP3 improves the insulin sensitivity of 3T3-L1 adipocytes by inhibiting inflammation and ameliorating insulin signalling transduction. Endokrynol Pol. 2014;65:252–8.
Yang L, Dong X, Zhang W. Astragaloside IV alleviates the brain damage induced by subarachnoid hemorrhage via PI3K/Akt signaling pathway. Neurosci Lett. 2020;735: 135227.
Hou M, Liu J, Liu F, Liu K, Yu B. C1q tumor necrosis factor-related protein-3 protects mesenchymal stem cells against hypoxia- and serum deprivation-induced apoptosis through the phosphoinositide 3-kinase/Akt pathway. Int J Mol Med. 2014;33:97–104.
Chen L, Qin L, Liu X, Meng X. CTRP3 alleviates Ox-LDL-induced inflammatory response and endothelial dysfunction in mouse aortic endothelial cells by activating the PI3K/Akt/eNOS pathway. Inflammation. 2019;42:1350–9.
Yang LH, Xu YC, Zhang W. Neuroprotective effect of CTRP3 overexpression against sevoflurane anesthesia-induced cognitive dysfunction in aged rats through activating AMPK/SIRT1 and PI3K/AKT signaling pathways. Eur Rev Med Pharmacol Sci. 2020;24:5091–100.
Acknowledgements
Funding
This work was funded by Science and Technology Project of Shenzhen (JCYJ20190807110803624), Science and Technology Project of Shenzhen Nanshan District (2020083) and Science and Technology Project of Shenzhen Baoan District (2019JD337). The rapid service fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Yue Zhang and Guangning Xu performed the experiments; Renqun Ye designed the study and wrote the manuscript; Baoyi Huang and Dongni Chen performed data analysis; all the authors approved the manuscript for submission.
Disclosures
Yue Zhang, Guangning Xu, Baoyi Huang, Dongni Chen and Renqun Ye have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on in vitro studies using cell lines and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhang, Y., Xu, G., Huang, B. et al. Astragaloside IV Regulates Insulin Resistance and Inflammatory Response of Adipocytes via Modulating CTRP3 and PI3K/AKT Signaling. Diabetes Ther 13, 1823–1834 (2022). https://doi.org/10.1007/s13300-022-01312-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01312-1