Abstract
Introduction
The fat and protein content can impact late postprandial glycemia; therefore, prolonged insulin boluses for high-fat/-protein meals are recommended for patients with type 1 diabetes on insulin pump therapy. It is not clear how to translate these findings to multiple daily injection (MDI) therapy. We hypothesized that regular insulin with a slower onset and a longer duration of action might be advantageous for such meals.
Methods
Twenty-five patients with well-controlled type 1 diabetes (mean HbA1c 6.8%, 51 mmol/mol, no episodes of hypoglycemia) on MDI therapy, aged 27.9 ± 4.3 years and well trained in flexible intensive insulin therapy, were given three test breakfasts with the same carbohydrate (CHO) content. The amount of fat and protein was low (LFP) or high (HFP). For LFP meals, patients received a rapid-acting insulin; for HFP meals, a rapid-acting or regular insulin was given in individual doses according to the CHO content and individual insulin-CHO ratios. Postprandial glycemia was determined by 6-h continuous glucose monitoring.
Results
Acute postprandial glucose levels measured for 2 h were similar after LFP and two HFP meals (7.8 ± 2.0, 8.1 ± 2.1, 8.0 ± 1.9 mmol/l). Late postprandial glycemia measured from 2 to 6 h was significantly lower after the LFP meal (6.7 ± 1.8 mmol/l, p < 0.05) than after the HFP meals, but there was no difference between the rapid-acting or regular insulin on HFP days (8.6 ± 2.6 and 8.9 ± 2.8 mmol/l, NS).
Conclusion
The preliminary results of this study indicate no benefit to cover fat-protein meals with regular insulin in individuals with type 1 diabetes treated with MDI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meal carbohydrate counting is widely used in individuals with type 1 diabetes on functional intensive insulin therapy treated with a basal-bolus insulin regimen by means of multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII) by insulin pumps. Advanced carbohydrate counting is recommended by official guidelines for type 1 diabetes treatment [1]. The prandial dose of rapid-acting insulin is based on the amount of carbohydrates in the meal to be consumed (in grams or carbohydrate exchangers) and an individualized insulin-to-carbohydrate ratio (ICR, units/g) [2]. In clinical practice, the meal carbohydrate amount is usually regarded as the most important determinant of the acute postprandial blood glucose level. Glycemia typically peaks within 60–90 min following a carbohydrate-based meal [3].
Recent studies have shown that the fat and/or protein content can impact late postprandial glycemia, and the effect of both nutrients is additive [4,5,6]. After a meal high in protein and fat added to a constant amount of carbohydrate, the peak of glycemia is similar in time but accentuated, and prolonged glucose excursions are observed from 3 to 6 h after the meal [6, 7]. These findings point to the need for prolonging insulin delivery with or without an increase in total mealtime insulin dosage [7,8,9,10,11]. The latest American Diabetes Association recommendations suggest that selected subjects with type 1 diabetes who have mastered carbohydrate counting should be educated on the impact of fat and protein on the glycemic profile [1].
All described strategies of combined carbohydrate, fat and protein counting and application of prolonged insulin boluses for mixed meals are appropriate for patients on CSII. Until now, there has been no attempt to solve the problem of adjustment of insulin for a meal with increased fat and protein content in patients treated with MDI who constitute the majority of patients with type 1 diabetes. The proposed but not verified methods for high-fat/high-protein meals in this group of patients are to inject an additional dose of rapid-acting insulin 1 h after the meal or to cover the meal with regular insulin [12, 13].
The main aim of study was to check if regular human insulin, which has a slightly later peak but also a longer total duration of activity, administered before a high-fat-protein meal, would give lower and more stable glycemia than a rapid-acting insulin analog used routinely in patients with type 1 diabetes treated with flexible MDI therapy. The additional aim was to evaluate whether the ICRs calculated in real life (typically for mixed meals) may lead to hypo- or hyperglycemia when used for carbohydrate or high-fat/protein meals.
Methods
This study included 25 patients with type 1 diabetes, 14 females and 11 males aged 20–40 years (mean 27.9 ± 4.3 years). The subjects were recruited from the Outpatient Clinic of the Department of Diabetology and Internal Diseases of Pomeranian Medical University in Szczecin. The inclusion criteria were functional intensive insulin therapy performed by multiple daily injections with pens (≥ 4 injections a day), disease duration longer than 12 months (to avoid the effect of remission) and good metabolic control (HbA1c within 3 months before the study < 8.0%, without episodes of severe hypoglycemia or unconscious hypoglycemia). The females included in the study were in the first phase of the menstrual cycle to avoid the influence of hormonal changes on glucose levels. All patients used a rapid-acting insulin before meals (aspart, lispro or glulisine) according to a regimen including an individual insulin-to-carbohydrate ratio appropriate for a meal at the time of day when the insulin dose was determined for the so-called carbohydrate exchange (CE) comprising 10 g of carbohydrates in the consumed meal. The basal insulin was used as a single injection in the evening hours at approximately 10:00 pm; 16 patients used a long-acting analog (glargine, detemir), and the remaining 9 people used NPH insulin. The exclusion criteria were gastrointestinal autonomic neuropathy, peripheral neuropathy, any kidney or liver disfunction, celiac disease, pregnancy and lactation, or other medical problems. The study design was approved by the Bioethics Committee of the Pomeranian Medical University (resolution no. KB-0012/42/10). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study. Since the measurement methods and insulin used in the study have been available for many years, the trial was not registered in the trial registration. On the 1st day of the study, after obtaining written informed consent to participate in the study, patients completed a questionnaire on their insulin doses and insulin-to-carbohydrate ratios. Height and weight were measured to calculate the body mass index (BMI), the waist and hip circumferences were measured to calculate the waist-to-hip ratio (WHR), and the blood pressure and pulse rate were measured. The presence of chronic complications of diabetes and comorbidities was determined based on the medical history, physical examination and analysis of the patients' medical records. Under basal conditions, fasting venous blood samples were collected for laboratory assessment of glycated hemoglobin HbA1c (HPLC method), lipid metabolism (total cholesterol, HDL and LDL of cholesterol, triglycerides), liver function (alanine aminotransferase, ALT) and renal function (creatinine, sodium, potassium, eGFR—calculated according to the MDRD formula). A sample of morning urine was collected to determine the albumin/creatinine ratio (ACR). The characteristics of the studied subjects are shown in Table 1.
Glucose Measurement
One day before administration of test meals, a continuous glucose-monitoring system for the retrospective evaluation of the glucose level was inserted (CGMS, iPro2, Medtronic Co., MiniMed, Northridge, CA, USA). The sensor was placed in the abdominal subcutaneous tissue. Each patient was trained in the use of CGMS and the proper calibration of the blood glucose meter. All patients used Contour Link blood glucose meters (Bayer, Basel, Switzerland). The patients were asked to refrain from intense physical activity on that day and all days of the study and from eating for 2 h before and 2 h after insertion of the iPro2 system to perform the calibration at maximally stabile glucose levels. According to the manufacturer recommendations, patients performed at least four glucose measurements per day, and the longest interval between two consecutive measurements could not exceed 12 h. To avoid the influence of injected insulin on CGMS measurements, administration of prandial insulin to subcutaneous tissue was recommended at a distance not less than 7.5 cm from the implanted sensor.
Test Meals
In the 3 consecutive days of the study, at breakfast time (6:30–8:00 a.m.), after the overnight fast, patients consumed a standardized meal ordered in one of the popular chain restaurants serving fast food meals. On the 1st day of the study, patients were given a carbohydrate meal with low fat and protein content (LFP), consisting of a roll (30 g carbohydrate, 2.5 g fat, 5.7 g protein; total of 165 kcal) and vegetable salad without dressing (75 g/12 kcal, vegetables: lettuce, cucumber, tomato; 1.5 g carbohydrate, 0.2 g fat, 1.1 g protein). Immediately before the meal, a rapid-acting insulin was injected into the abdomen subcutaneous tissue. The prandial insulin was given in individual doses according to the CHO content, individual ICR and preprandial glucose level (individual correction ratio). On the 2nd and 3rd days of the study, patients ate a meal rich in fat and protein (HFP), i.e., a hamburger, bun and salad (12 kcal). The hamburger consisted of the same amount of carbohydrates as on the 1st day, steak meat, cheese and sauces (30 g carbohydrates, 37 g fat, 30 g protein, a total 573 kcal). On the 2nd day, the patients administered a rapid-acting insulin analog in the dose calculated as on the 1st day. On the 3rd day, regular human insulin was administered in the same dose as that administered on days 1 and 2. Human insulin was injected in a standard way 30 min before meals. The type of regular insulin was determined by the type of rapid-acting insulin used previously. For example, instead of lispro insulin, Humulin R insulin (Eli-Lilly) was used, and instead of aspart insulin, Actrapid was used (NovoNordisk). The carbohydrate content and the glycemic index of the meals remained constant on each day of the study. Patients consumed breakfast meals in 15 min, and they drank 250 ml of non-carbonated mineral water. To ensure similar conditions for all participants and to minimize physical activity, patients stayed sedentary for a 6-h postprandial period in the Outpatient Clinic. The study was conducted under the supervision of a clinical dietitian. On the last day of the study, the iPro2 system was disconnected, and CareLink reports with recorded glucose levels were generated. Glucose levels were analyzed for a 6-h postprandial period of three test meals.
Statistical Analysis
The values of quantitative variables are presented as the arithmetic means with standard deviations (SD). Glucose concentrations were compared using paired Student’s t test. Values of the time from the meal to maximum glucose concentration were compared using the non-parametric Wilcoxon signed-rank test since their distribution was significantly different from normal distribution. Two-way repeated-measures ANOVA was performed with time from the HFP meal, insulin type and their interaction term as independent variables and glucose concentration as the dependent variable. Statistical analysis was performed using the STATISTICA 13 program (StatSoft, Poland). The level of significance was set at p < 0.05.
Results
Fasting glucose levels measured in the morning, just before a test meal, were similar for each day of the study (7.2 ± 2.2, 6.9 ± 2.2, 7.1 ± 1.9 mmol/l). Postprandial glycemia, evaluated at half-hour intervals, did not differ significantly for the first 2 h of the observation, regardless of whether it was a carbohydrate meal with LFP content consumed on the 1st day or an HFP meal consumed on the 2nd and 3rd days of the study (Table 2). The type of insulin used for the HFP meal also did not affect the trend in glucose levels in successive intervals of the first 120 min of the study. Two hours after the carbohydrate meal consumed on the 1st day, six patients experienced mild symptomatic hypoglycemia, which resulted in the oral administration of additional carbohydrates and exclusion from further observation on that day of the study. In the group of patients who experienced hypoglycemia, four were treated with a long-acting analog and two with NPH insulin. Due to the exclusion of the subjects with hypoglycemia from further observation, postprandial glycemia between 120 and 360 min on the 1st day of the study was analyzed for 19 participants. On the 2nd and 3rd days of the study, after the HFP meals, hypoglycemia did not occur in any patients (Fig. 1).
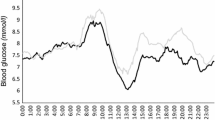
Mean glucose levels within 6 h after eating an HFP meal on days 2 and 3 were significantly higher than after the LFP meal on day 1 of the study (Fig. 2). Glucose levels after meals enriched in fat and protein were similar regardless of the type of the administered insulin (rapid-acting insulin on day 2 vs. regular insulin on day 3). The type of meal eaten and the insulin used had no significant effect on the average glucose levels in the first 2 h of the observation (7.8 ± 2.0, 8.1 ± 2.1, 8.0 ± 1.9 mmol/l). Late postprandial glucose levels between 2 and 6 h were significantly lower after the LFP meal than after the HFP meals (6.7 ± 1.8; 8.6 ± 2.6, 8.9 ± 2.8 mmol/l, respectively, p < 0.05).
The maximum average blood glucose level after the LFP meal was 8.4 ± 2.3 mmol/l and was achieved in the 90th minute of the study, after which it steadily decreased, reaching the lowest value of 6.3 ± 1.8 mmol/l in the 240th and 270th minute of the study. In the 360th minute of the study, the mean value of 6.9 ± 1.9 mmol/l was similar to the fasting level observed on that day. The average blood glucose level after the first HFP meal rose to 8.8 ± 2.4 mmol/l at the 90th minute, was approximately 8.9 ± 2.8 mmol/l in the 150-210th minute and remained relatively stable until the end of the study. At the 360th minute, the average blood glucose was 8.3 ± 3.1 mmol/l. Postprandial values after the second HFP meal were almost identical. The analysis of time from the HFP meal to the maximum glucose concentration showed a tendency to longer values for regular human insulin compared to rapid-acting insulin (median 270 vs. 150 min), but the difference did not reach statistical significance (p = 0.073).
Two-way repeated-measures ANOVA considering all glucose concentration data from two HFP meals in one model confirmed that glycemia changed significantly after meals (p < 0.00001), but was not associated with the type of insulin used (p = 0.61), and there was no interaction between the glycemia profile and type of insulin (p = 0.48).
Discussion
The vast majority of studies conducted thus far evaluating the dosage of insulin required for high-fat and high-protein meals consumed by patients with type 1 diabetes were performed in children and young people, and the test meal usually consisted of pizza with high or very high fat and protein content consumed at dinner or, less frequently, lunchtime. Occasionally, the studies were conducted in adult patients, but then they included very small groups of subjects [4, 14]. Only a single study has evaluated postprandial glycemia at breakfast time [6]. The surveyed patients were usually treated with insulin pumps or occasionally formed subgroups treated with multiple injections, which were not analyzed separately [6, 15].
In our study, we evaluated a group of young adult patients with type 1 diabetes treated with insulin by multiple injections, and the test meal was served at breakfast time. All patients were well controlled (mean HbA1c 6.8%, 51 mmol/mol) and well trained in flexible intensive insulin therapy, calculating the mealtime insulin doses based on the carbohydrate content of the meals. The meal was served for breakfast, after an overnight fast, to avoid the potential impact of a previous meal. A fast-food mixed meal with increased fat and protein content was similar to the composition of meals eaten often in real life at this time of day. It was carefully standardized in terms of nutrients and contained 31.5 g carbohydrates, 37.2 g fat, 31.1 g protein and 585 kcal. An individual dose of insulin was calculated for the carbohydrate content and current insulin-to-carbohydrate ratio appropriate for breakfast in a given patient. The dose of insulin and amount of carbohydrates in the meals remained constant on the subsequent days. While the use of pure protein preparations in an amount of approximately 20 g, or even 50 g, does not appear to affect the course of glycemia [5, 16], an increase in the amount of fat and protein in a mixed meal containing carbohydrates may cause a late rise of postprandial glycemia, and the hyperglycemic effect of fat and protein is additive [6]. The timing of assessment of postprandial glycemia, measured by a CGM, was set at 6 h, as precision tests using a closed-loop glucose control in adults with type 1 diabetes have shown that after a high-fat meal, the need for insulin begins to rise after 2 h up to a maximum value between 4 and 6 h [4]. The maximum values of glycemia 6 h after a high-fat-protein meal were also observed by other authors [15]. It is assumed that the late increase in postprandial glycemia after a high-fat, high-protein meal may be the result of a slower emptying of the stomach related to the fat content and gluconeogenesis induced by amino acids [17, 18].
Thus far, authors studying the problem of increased insulin requirements for high-fat, high-protein meals have sought solutions only for patients using insulin pumps (prolonged or complex boluses). It cannot be excluded that the beneficial effect on postprandial glycemia could mainly be due to an increase in the insulin dose added for proteins and fats rather than the type of bolus—simple or complex [7]. However, when the dose of insulin was increased for protein and fat content, significantly more episodes of hypoglycemia were observed [7, 11]. Some data suggest that a simple bolus of insulin calculated on the basis of the carbohydrate content and individual insulin-to-carbohydrate ratio administered for a meal with an average fat and protein content gives satisfactory results [8]. This ratio, although it theoretically only takes carbohydrates into account, in clinical practice is calculated for mixed meals also containing fat and protein. It is therefore likely that, except for meals with a very high content of fat and protein for which the insulin dosage should be increased (it is unclear how much), for the meals with an average fat and protein content, there is no need to correct (increase) the insulin dosage.
Here, for the first time to our knowledge, we compared the impact of regular human insulin and a short-acting insulin analog given for a high-fat/high-protein meal on postprandial glycemia in subjects with type 1 diabetes treated with insulin by multiple daily injections. The course of glycemia for the first 1.5 h was almost identical and remained similar at the 2nd hour of observation, regardless of the type of meal and insulin administered. Despite the expectations, the type of insulin used had no effect on the course of postprandial glycemia after high-fat and protein meals evaluated by retrospective CGMS. Differences were not observed for either early or late glycemia. The glucose levels after 2 h and until the end of the study were significantly higher than for intake of a meal with the same carbohydrate content but low in fat and protein. This observation has an important practical aspect, as for meals with high fat and protein contents, glycemia measured before the next meal, about 6 or 8 h after the previous one, may be too high, even despite good levels measured after 1.5 or 2 h, typically regarded as the postprandial peak. This situation may suggest the correct dose of prandial insulin and a too low dose of basal insulin, which in turn may lead to incorrect treatment decisions.
The fact that the type of insulin used for meals had no effect on the postprandial glycemia profile is difficult to explain. Perhaps some differences in glycemia would be revealed after more than 6 h of observation. It seems that the next step in the study of glycemia after high-fat and protein meals should be to try use a small increase in the dose of both types of prandial insulin and to prolong the observation period over 6 h. It would also be interesting to compare the effect of these two different prandial insulins administered for meals with higher fat and protein contents than in our study.
The hypothesis that the insulin-to-carbohydrate ratio used in practice also includes fat and protein and may be overestimated for carbohydrate meals seems to be confirmed by the observation from the 1st day of the study when symptomatic, although mild, hypoglycemia occurred in 6 of 25 subjects, which was the cause of their exclusion from further observation on that day of the study. It was surprising that the hypoglycemic episodes occurred 2 h after the carbohydrate meal with a very low fat and protein content, whereas the course of the glycemia for the first 2 h was similar between meals. In 19 subjects with no symptoms of hypoglycemia, mean glucose levels between 2.5 and 6 h on the 1st day of observation were also quite low and averaged 6.7 mmol/l. It can be assumed that the empirically calculated insulin-to-carbohydrate ratio, which worked in everyday life for mixed meals also containing fat and protein, was too high for the carbohydrate meal. Similar observations have been made by other authors. In a study by Smart et al., after a low-fat and low-protein meal such as the one used in our study with 30 g of carbohydrates, 14 episodes of hypoglycemia occurred after 5 h, whereas after a meal with a higher content of fat (35 g) and protein (40 g), with the same amount of carbohydrates, there was only one episode of hypoglycemia, although the doses of insulin were not changed (6). Hypoglycemia also occurred in 9 of 15 subjects studied by Neu et al. when a routinely calculated dose of insulin was applied for a standard meal containing 70 g carbohydrates in which the fat and protein content was lowered [15]. It is highly probable that the so-called standard meal used in our study contained less fat and protein than the food used by patients in real life for which the empiric insulin-to-carbohydrate ratio had been previously calculated. It appears that for the same reason, the increase in the dose of insulin for meals with a higher content of fat and protein (pizza) has proved to result in numerous hypoglycemic episodes [7, 11, 19].
The lack of random order of the eaten meals and giving prandial insulin are limitations of our study. On the 1st and 2nd day of the study we decided to maintain the same insulin as was used by patients in real life. It was administered for two different meals (a carbohydrate or fat/protein meal), with the expectation of making the course of the study more interesting and thus keeping participants in the study. Moreover, the 1st day was a “control day” for day 2 when patients consumed fat/protein meals. Because regular human insulin is not typically used for meals in type 1 diabetes, we decided to give it for the fat/protein meal on the 3rd (last) day. As all procedures were exactly the same for all patients and carefully supervised, we suppose that the obtained results are quite reliable. Another limitation of our study was the relatively small sample of investigated subjects; however, the number of participants is very similar to the samples in other trials investigating this problem, which had 26–33 or even 7 subjects [4,5,6,7]. Such studies are complex, time consuming and difficult to conduct in young, usually very active subjects with type 1 diabetes.
Conclusion
The data from our study may have very practical value. We tried to resolve the problem of prolonged hyperglycemia observed in subjects with type 1 diabetes treated with pens after the typical high fat/high protein meals served in fast food restaurants and eaten very often by young people. They indicate no benefit to covering mixed carbohydrate fat/protein meals with regular insulin in individuals with type 1 diabetes treated with multiple daily injections. As there was no benefit to covering such meals with regular insulin, another way to deal with this problem could be suggested. Maybe it would be enough to increase the dosage of the rapid-acting insulin analog, taking into account the calorie/energy content of the meal. However, the required increase in the insulin dosage for high-fat/protein meals in subjects with type 1 diabetes staying on MDI pen therapy has not been precisely established. The study results indicate the need to reduce the empirical insulin-to-carbohydrate ratio when eating a carbohydrate meal with a very small amount of fat and protein.
References
American Diabetes Association. Pharmacologic approaches to glycemic treatment. Sec. 8. In Standards of Medical Care in Diabetes 2017. Diabetes Care. 2017;40(Suppl. 1):S64–74.
DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomized controlled trial. BMJ. 2002;325:746–9.
Smart CE, King BR, McElduff P, Collins CE. In children using intensive insulin therapy, a 20 gram variation in carbohydrate amount significantly impacts on postprandial glycaemia. Diabet Med. 2012;7:e21–4.
Wolpert HA, Atakov-Castillo A, Smith S, Steil GM. Dietary fat acutely increase glucose concentrations and insulin requirements in patients with type 1 diabetes. Diabetes Care. 2013;36:810–6.
Paterson MA, Smart CEM, Lopez PE, et al. Influence of dietary protein on postprandial blood glucose levels in individuals with type 1 diabetes mellitus using intensive insulin therapy. Diabet Med. 2016;33:592–8.
Smart CE, Evans M, O’Connell S, et al. Both dietary protein and fat increase postprandial glucose excursions in children with type 1 diabetes, and the effect is additive. Diabetes Care. 2013;36:3897–902.
Pańkowska E, Błazik M, Groele L. Does the fat–protein meal increase postprandial glucose level in type 1 diabetes patients in insulin pump: the conclusion of a randomized study. Diabetes Technol Ther. 2012;14:16–22.
De Palma A, Giani E, Iafusco D, et al. Lowering postprandial glycemia in children with type 1 diabetes after Italian pizza “margherita” (TyBoDi2 Study). Diabetes Technol Ther. 2011;13:483–7.
Chase HP, Saib SZ, MacKenzie T, Hansen MM, Garg SK. Post-prandial glucose excursions following four methods of bolus insulin administration in subjects with type 1 diabetes. Diabet Med. 2002;19:317–21.
Jones SM, Quarry JL, Caldwell-McMillan M, Mauger DT, Gabbay RA. Optimal insulin pump dosing and postprandial glycemia following a pizza meal using the continuous glucose monitoring system. Diabetes Technol Ther. 2005;7:233–40.
Kordonouri O, Hartmann R, Remus K, Bläsig S, Sadeghian E, Danne T. Benefit of supplementary fat plus protein counting as compared with conventional carbohydrate counting for insulin bolus calculation in children with pump therapy. Pediatric Diabetes. 2012;13:540–4.
Bell KJ, Smart CE, Steil GM, Brand-Miller JC, King B, Wolpert HA. Impact of fat, protein and glycemic index on postprandial glucose control in type 1 diabetes: implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care. 2015;38:1008–15. https://doi.org/10.2337/dc15-0100.
Paterson M, Bell KJ, O’Connell SM, Smart CE, Shafat A, King B. The role of dietary protein and fat in glycaemic control in type 1 diabetes: implications for intensive diabetes management. Curr Diab Rep. 2015;15:61. https://doi.org/10.1007/s11892-015-0630-5.
Lee SW, Cao M, Sajid S, et al. The dual-wave bolus feature in continuous subcutaneous insulin infusion pumps controls prolongued postaprandial hyperglycemia better than standard bolus in type 1 diabetes. Diabetes Nutr Metab. 2004;17:211–6.
Neu A, Behret F, Braun R, et al. Higher glucose concentrations following protein- and fat-rich meals—the Tuebingen Grill Study: a pilot study in adolescents with type 1 diabetes. Pediatr Diabetes. 2015;16:587–91.
Klupa T, Benbenek-Klupa T, Matejko B, Mrozinska S, Malecki MT. The impact of a pure protein load on the glucose levels in type 1 diabetes patients treated with insulin pumps. Int J Endocrinol. 2015;2015:216918. https://doi.org/10.1155/2015/216918.
Lodefalk M, Aman J, Bang P. Effects of fat supplementation on glycaemic response and gastric emptying in adolescents with type 1 diabetes. Diabet Med. 2008;25:1030–5.
Linn T, Geyer R, Prassek S, Laube H. Effect of dietary protein intake on insulin secretion and glucose metabolism in insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81:3938–43.
Pańkowska E, Błazik M. Bolus calculator with nutrition database software, a new concept of prandial insulin programming for pump users. J Diabetes Sci Technol. 2010;4:571–6.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval to the version to be published.
Prior Presentation
The data were partially presented at the 17th Congress of the Polish Diabetes Association in Kielce 5–7 May 2016; reference: Diabetol. Klin., 2016; 5: suppl. B, B48 (abstract).
Disclosures
Karolina Jabłońska, Piotr Molęda, Krzysztof Safranow and Lilianna Majkowska have nothing to disclose.
Compliance with Ethics Guidelines
The study design was approved by the Bioethics Committee of the Pomeranian Medical University (Resolution No. KB-0012/42/10). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Data Availability
The data sets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/FF1DF060749E3F98.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jabłońska, K., Molęda, P., Safranow, K. et al. Rapid-acting and Regular Insulin are Equal for High Fat-Protein Meal in Individuals with Type 1 Diabetes Treated with Multiple Daily Injections. Diabetes Ther 9, 339–348 (2018). https://doi.org/10.1007/s13300-017-0364-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-017-0364-2