Abstract
Introduction
Glucagon-like peptide-1 (GLP-1) has been the focus of considerable research activity in the treatment of type 2 diabetes mellitus (T2DM) because the incretin effect is significantly reduced or absent in individuals with T2DM. Thus, pharmacologic efforts to develop medications that mimic the actions of GLP-1 have become a target for improving or reversing chronic hyperglycemia. Two GLP-1 receptor agonists are commercially available: exenatide twice daily (b.i.d.) and liraglutide once daily (q.d.). Targeted and individualized intensification of diabetes management can best be accomplished with a thorough understanding of these new medications.
Methods
Information was gathered through a search of MEDLINE and PubMed for GLP-1 and glycemic management in patients with type 2 diabetes.
Results
Activation of the GLP-1 receptors on the β-cells results in enhanced levels of insulin biosynthesis, β-cell proliferation, resistance to β-cell apoptosis, and enhanced β-cell survival in both humans and rodents; yet, the risk of hypoglycemia is minimized because insulin production and exocytosis occurs in a glucose-dependent manner. The efficacy and safety of the two commercially available GLP-1 receptor agonists, liraglutide and exenatide, in managing postprandial glycemia have been well documented in numerous clinical trials, in which reductions in glycosylated hemoglobin (HbA1c) levels of −0.79% to −1.12% have been demonstrated. Weight reduction/maintenance and improvements in blood pressure and lipidemia have also been reported.
Conclusion
Because GLP-1 receptor agonists work in a glucose-dependent manner, they are likely to reduce hyperglycemia safely, without a marked fluctuation toward hypoglycemia. In the process of acutely restoring β-cell function, GLP-1 agonists may allow patients to achieve HbA1c <7% without experiencing weight gain or hypoglycemia. The ability of GLP-1 receptor agonists to improve blood pressure and postprandial lipidemia in the context of weight neutrality or weight loss may have the potential to ameliorate some of the cardiovascular risks observed in patients with T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the 1960s, data suggested that oral glucose elicited a much greater secretion of insulin than a similar amount of glucose administered intravenously,1 and that this potentiation of insulin secretion by the gut may be responsible for up to 70% of the insulin response to a meal.2,3 This physiologic activity was subsequently referred to as the intestinal secretion of insulin, or incretin effect. It was later found that two hormones, gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), are responsible for the incretin effect.3
GLP-1 and GIP have been the focus of considerable research activity in the treatment of type 2 diabetes mellitus (T2DM) because the incretin effect is significantly reduced or absent in people with T2DM.4 The reduced incretin effect is believed to contribute to impaired regulation of insulin and glucagon secretion in these patients.4
Although secretion of GIP remains relatively normal in T2DM subjects, its effect on insulin secretion is severely impaired.2,3 Conversely, despite the reduced secretion of GLP-1, its insulinotropic and glucagon-suppressive actions remain intact.2 In addition, there is a high concordance between the physiologic actions of GLP-1 and the therapeutic needs of patients with T2DM; the effects of GLP-1 on insulin and glucagon secretion following the ingestion of a meal are glucose dependent,3 thereby providing protection against hypoglycemia. Thus, pharmacologic efforts to develop medications that mimic the actions of GLP-1 have become a target for improving chronic hyperglycemia.
Two GLP-1 receptor agonists are now commercially available: exenatide twice daily (b.i.d.) and liraglutide once daily (q.d.). When given by subcutaneous injection with pen devices, these medications become receptor bound and result in actions similar to the native hormone. Targeted and individualized intensification of diabetes management may best be accomplished with a thorough understanding of the currently available pharmacotherapeutic interventions. Clinicians should not only consider an agent’s mechanism of action, efficacy, safety, and tolerability, but also how to proactively address patient concerns related to the introduction of any new medication. The goal of this clinical review is to discuss the differences between the two medications in order to help clinicians make appropriate decisions regarding their use in patients with T2DM.
The Physiologic Actions of Incretin Hormones
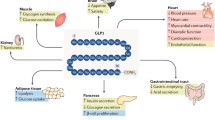
Both GIP and GLP-1 are secreted from gut endocrine cells in response to meals, and exert their actions by binding to structurally distinct receptors. The GIP receptor is predominantly expressed in pancreatic islet β-cells, whereas GLP-1 receptors are expressed in islet α- and β-cells, as well as in the central and peripheral nervous systems, heart, lung, kidney, and gastrointestinal tract.5
Activation of the GIP and GLP-1 receptors on the β-cells results in insulin production and exocytosis in a glucose-dependent manner (ie, only during hyperglycemia), which minimizes risk of hypoglycemia.5 Sustained activation of these receptors results in enhanced levels of insulin biosynthesis, β-cell proliferation, resistance to β-cell apoptosis, and enhanced β-cell survival in both humans and rodents.5 Table 1 presents the glucose homeostatic actions of both GIP and GLP-1 in an euglycemic individual.2,5,6 Shortly after the incretin hormones are secreted, they are rapidly inactivated by dipeptidyl peptidase-4 (DPP-4), leaving only 20% of the endogenously secreted GLP-1 to induce insulin secretion from pancreatic β-cells.7
Exogenous GLP-1 administered drugs and DPP-4 inhibitors have distinct mechanistic actions. The physiologic and pharmacologic effects of GLP-1 are based on the concentration of GLP-1 or a GLP-1 receptor agonist, as well as the affinity of receptor binding and number of receptors bound; the more receptors that are bound and activated, the greater the glycemic and nonglycemic responses to a given class of medications. The DPP-4 inhibitors, however, do not bind to GLP-1 receptors; rather, they prolong the half-life of endogenous GLP-1, thereby increasing GLP-1 levels.8 Thus, GLP-1 agonists have greater effects on glycemic control, weight loss, and gastric emptying, compared with the DPP-4 inhibitors (ie, sitagliptin and saxagliptin), which are also commercially available.9
Differentiating the GLP-1 Agonists
Successful treatment of T2DM requires an understanding of the disease pathogenesis, as well as a willingness to individualize and appropriately intensify therapy. As such, clinicians should always consider treatment as soon as possible, at as low a dose as possible, and as safely, as long, and as rationally as possible. In contrast to many other antidiabetic agents that increase the risk of hypoglycemia and/or weight gain, weight loss occurs with GLP-1 agonists while the risk of hypoglycemia is low, unless combined with an insulin secretagogue, such as a sulfonylurea. Within the class of GLP-1 agonists, multiple pharmacologic differences exist that may influence which medication is ultimately prescribed for a given individual. To address these differences, the two commercially available GLP-1 agonists—exenatide and liraglutide—will be discussed according to the systems that are affected by their use.
Pharmacologic Profiles
Both exenatide b.i.d. and liraglutide, q.d. were developed to resist DPP-4 degradation and both, therefore, have a protracted mechanism of action. Exenatide shares 53% of its amino acid sequence with native GLP-1.10 Exenatide has a time to peak plasma concentration (Tmax) of 2 to 3 hours, a terminal elimination half-life (t½) of 3.4 hours on day 1, with a corresponding peak plasma concentration (Cmax) of 163 pg/mL.11 Exenatide must be injected subcutaneously b.i.d., within 60 minutes prior to a meal.
By contrast, the degree of sequence identity between liraglutide and native GLP-1 is high, at 97%.12 Although it has been speculated that self-association is the primary mechanism behind the delayed absorption seen in liraglutide, reversible binding to albumin in the bloodstream and increased metabolic stability appear to be the basis for its prolonged half-life and delayed degradation by DPP-4.13 With a t½ of 11.6 to 12.8 hours and a Tmax of 10 to 14 hours, liraglutide is suitable for q.d. dosing via subcutaneous injection, without regard for meals.14
Exenatide is approved for monotherapy or as part of combination therapy (as an adjunct to diet and exercise). Exenatide is approved as an add-on to metformin, sulfonylurea, thiazolidinedione, a combination of metformin and sulfonylurea, or a combination of metformin and a thiazolidinedione. Liraglutide is indicated as monotherapy in patients who are metformin intolerant, or for patients in whom metformin may be contraindicated. Liraglutide may also be used in combination with metformin, metformin plus a sulfonylurea, metformin plus a thiazolidinedione (pioglitazone), or a sulfonylurea plus a thiazolidinedione. Patients using liraglutide in combination with a sulfonylurea should have the dose of the sulfonylurea reduced to minimize their risk of hypoglycemia.
Clinical Efficacy of Liraglutide and Exenatide
The efficacy and safety of liraglutide and exenatide in clinical trials have been well documented in the Liraglutide Once Daily Compared with Exenatide Twice Daily (LEAD-6) study, a 26-week, randomized, open-label, parallel-group, multinational trial, conducted in 132 centers across 15 countries, including Europe and the United States.15 A total of 464 patients with T2DM were randomized to receive liraglutide 1.8 mg q.d. subcutaneously or exenatide 10 μg b.i.d. subcutaneously, for 26 weeks.15 All patients were maintained with background oral antidiabetic treatment, which included maximally tolerated doses of metformin and/or sulfonylurea.15
The primary efficacy endpoint was change in glycosylated hemoglobin (HbA1c) value from baseline to week 26. Secondary efficacy endpoints included the proportion of patients reaching target HbA1c values (>7.0% and 6.5%), changes in fasting plasma glucose levels, self-measured 7-point plasma glucose profiles, body weight, β-cell function, glucagon level, blood pressure, and lipid profiles.15 Results from the study indicate that liraglutide q.d. provides significantly improved glycemic control compared with exenatide b.i.d., with only minimal and transient adverse events (AEs) (Table 2).15 A reduction in HbA1c level was significantly greater with liraglutide (−1.12%), compared with exenatide (−0.79%; P<0.001).15 In addition, a significantly greater proportion of patients receiving liraglutide compared with exenatide reached an HbA1c level of ≤7.0% (54% vs. 43%, respectively; P=0.0015), as well as an HbA1c level of ≤6.5% (35% vs. 21%, respectively; P<0.001).15
Significant reductions in fasting plasma glucose levels from baseline were also observed with liraglutide (−29 mg/dL) compared with exenatide (−11 mg/dL; P<0.001); postprandial glucose reductions after breakfast and supper were significantly greater with exenatide than with liraglutide (difference after breakfast of 23.96 mg/dL, P=>0.001; difference after supper, 18.2 mg/dL, P=0.005).15 The homeostasis model assessment of β-cell function (HOMA-B), which is utilized to quantify β-cell function, was significantly greater for the liraglutide group (32.12%) than with the exenatide group (2.74%; P<0.001).15 Changes in body weight were comparable and clinically meaningful with both medications (liraglutide −3.24 kg vs. exenatide −2.87 kg; P=0.224);15 approximately the same percentage of patients on each medication lost weight (liraglutide 78% vs. exenatide 76%).15 A significantly lower rate of minor hypoglycemia was observed with liraglutide (1.932 events) than with exenatide (2.600 events; P=0.013). The incidence of nausea was initially comparable between the two treatment groups, but was followed by a trend toward more rapid resolution of nausea in the liraglutide group, such that by week 26, only 3% of patients on liraglutide experienced nausea, versus 9% on exenatide (P<0.001).15
During a 14-week extension of the LEAD-6 trial, patients switched from exenatide 10 μg b.i.d. to liraglutide 1.8 mg q.d., or continued on liraglutide 1.8 mg q.d.16 Overall, conversion from exenatide to liraglutide was well tolerated, and further improved parameters of glycemic control.16 More specifically, by study week 40, patients who switched from exenatide to liraglutide experienced further and significant reductions in HbA1c levels (−0.32%; P<0.001), fasting plasma glucose (−16 mg/dL; P<0.001), and body weight (−0.9 kg; P<0.001).16 Furthermore, patients who continued on liraglutide experienced further reductions in body weight (−0.4 kg).16 The greater efficacy of liraglutide may be secondary to sustained GLP-1 receptor activation over 24 hours via q.d. dosing of liraglutide, compared with the biphasic levels that occur with the b.i.d. dosing schedule of exenatide.16
From a clinical standpoint, clinicians should understand how to minimize the occurrence of nausea for either GLP-1 agonist. In many of the clinical trials, patients were “forced titrated” rapidly upwards towards the medication’s maximum therapeutic dose. If the patients experienced any nausea they were offered few options other than continuing in the study, with the understanding that the nausea would be transitory, or simply withdrawing consent and dropping out of the study voluntarily. In five 26-week registration trials, approximately 13% of liraglutide-treated patients experienced some nausea within the first 2 weeks; however, only 2.8% of all subjects withdrew from these studies due to nausea.12 By comparison, in clinical trials of metformin, 25.5% of patients experienced nausea, compared with 8.3% who received placebo.17
The package inserts for exenatide and liraglutide do not provide suggestions for how to educate patients on minimizing nausea when initiating either medication. Therefore, we will provide several tools used in our clinical practices that may be helpful in counseling patients about management of nausea associated with exenatide and liraglutide.
Firstly, patients must be made aware that nausea is a very common adverse event (AE) with GLP-1 agonists. However, nausea, if it occurs, is likely to be transient and mild. Secondly, it should be explained to patients that GLP-1 agonists make patients feel full. They may not feel hungry when using these medications. If they attempt to eat and challenge their satiety, nausea and vomiting will likely occur. Thirdly, if nausea does occur, the process of up-titration of medication dosages should be slowed down. More specifically, patients may remain on the exenatide 5-μg dose for longer than 1 month, or on the liraglutide 0.6-mg dose for longer than 1 week, if necessary, before dose escalation. If a patient complains that the medication is causing excessive nausea or they refuse to continue using the medication for more than a few days, the patient should be evaluated for a possible eating disorder. In our experience, some patients with T2DM live to eat; any medication that minimizes their appetite would not be acceptable with their lifestyle and will be quickly rejected.
Immunogenicity
GLP-1 analogs are peptides and, therefore, antibody formation may occur that potentially results in injection site reactions, loss of glycemic control, and anaphylaxis. In registry trials, antibodies that had a neutralizing effect on liraglutide in an in-vitro assay occurred in 2.3% of the liraglutide-treated patients in a 52-week monotherapy trial, and in 1.0% of the liraglutide-treated patients in 26-week add-on combination therapy trials; however, none of these individuals experienced deterioration of glycemic control.12
In the 30-week registry trials for exenatide, low titer antiexenatide antibodies, which did not affect glycemic control, were detected in 38% of patients during the phase 3 development program.18 However, an additional 6% of patients had very high antibody titers at 30 weeks; half of these patients (3%) had an impaired glycemic response.18
From a clinical standpoint, the true significance of antibody induction is unclear. Antibodies to therapeutic proteins may compromise efficacy by neutralizing the medication and/or triggering AEs, ranging from mild injection site reactions to life-threatening anaphylaxis. Therapeutic proteins with higher structural similarity to endogenous proteins generally have a lower risk of both antibody formation and high antibody titer development.19 Liraglutide shares 97% homology to human GLP-1,12 compared with exenatide, which has 53% shared homology.10
In the LEAD-6 head-to-head study and the open-label extension arm, comparing the safety and efficacy of liraglutide and exenatide, antibody titers were obtained at weeks 0, 12, 26, 40, 41, 78, and 79, prior to the administration of the daily dose.20 After 78 weeks on liraglutide, four (2.6%) of 154 patients had low-titer antibodies. In the four patients who developed neutralizing antibodies against liraglutide, HbA1c levels were reduced by up to 1.9% from baseline over the 78 weeks.20 After the 26-week exenatide study, 113 (61%) of 185 patients developed antibodies to the medication.20 Those patients who had “high titers” of neutralizing antibodies demonstrated a minimal reduction in their HbA1c level (−0.1%), compared with those individuals who had “low titers” of neutralizing antibodies, and were able to reduce their HbA1c by 1.0%.20 In the LEAD-6 extension protocol, 1% of the liraglutide-to-liraglutide patients had injection site reactions, which were described as “irritation” at the injection site. Approximately 2% of the exenatide-to-liraglutide patients experienced injection site reactions;20 75% of these reactions occurred when the exenatide antibody was positive.20
All of the above adverse reactions were mild, and patients recovered and continued in the trial. The LEAD-6 trial, therefore, demonstrates that the presence of neutralizing antibodies may minimize the efficacy of a medication. However, patients with high antibody titers who were switched to liraglutide did not appear to experience a compromise in their glycemic response. For liraglutide-treated patients, the presence of neutralizing antibodies appears to have minimal clinical significance.
Effects on Islet β-Cell Function
In animal and in-vitro studies, incretin hormones have been shown to inhibit β-cell apoptosis and increase β-cell proliferation.21 Acutely, incretin therapy improves β-cell function and glycemia. With chronic use of incretin hormones, the potential exists to possibly reverse or stabilize the hyperglycemic disease process.
In 2005, Fehse and colleagues demonstrated that patients with T2DM who were pretreated with a single intravenous infusion of exenatide, followed by a bolus injection of glucose, had a first-phase and second-phase insulin secretory pattern equal to that of healthy subjects.22 Similar effects on β-cell stimulated insulin secretion were noted when liraglutide was injected subcutaneously.23 Interestingly, the liraglutide subjects had nearly normalized β-cell secretory output of insulin after a single injection of liraglutide, compared with individuals who received placebo.23
Longer-term effects of GLP-1 receptor agonists on β-cell function have been studied with liraglutide. In a randomized controlled clinical trial, 39 patients with T2DM and a baseline HbA1c of 8.1 to 8.5% (depending on the treatment group), were given liraglutide at doses of 0.65, 1.25, or 1.9 mg per day versus placebo for 14 weeks.24 The HbA1c levels in the liraglutide patient groups decreased by 1.0% to 1.5%, compared with placebo (P<0.05).24 In addition, the 0.125 mg and 1.9 mg doses of liraglutide increased first-phase insulin secretion, compared with placebo, by 118% and 103%, respectively (P<0.05); second-phase insulin response was significantly increased only in the liraglutide group receiving 1.25 mg per day (P=0.005 vs. placebo).24
To date, no human data have been presented to suggest that incretin-based therapy that protects or restores β-cell mass or function is durable. Thus, as in the case of exenatide, the restoration and improvement in β-cell function appears to be apparent only for as long as the medication is being utilized.25
Effects on Cardiovascular Markers
Positive effects on cardiovascular markers have been noted for both exenatide and liraglutide in multiple randomized, double-blind, placebo-controlled studies. However, there have been no studies establishing conclusive evidence of macrovascular risk reduction with any GLP-1 agonist. Although meta-analysis of the six trials comparing liraglutide 1.8 mg q.d. with agents commonly used in the treatment of T2DM (ie, glimiperide, rosiglitazone, and insulin glargine) and exenatide have assessed the impact of the incretin therapies on cardiovascular risk markers (Table 3), it is uncertain whether use of GLP-1 agonists will have a substantial impact on cardiovascular outcomes.26 However, preliminary analysis of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study showed a 75% reduction in mortality in exenatide-treated patients.27
Both liraglutide and exenatide exert a positive effect on blood pressure that appears to be independent of weight reduction. In one study evaluating the effects of ≥3 years of exenatide therapy in patients with T2DM (n=151), a sustained reduction in both diastolic blood pressure (mean change from baseline, −3.3 mmHg) and in systolic blood pressure (mean change from baseline, −3.5 mmHg) were reported.28 With liraglutide, systolic blood pressure reduction occurs to a greater extent than reduction in diastolic blood pressure. In one 14-week study, systolic blood pressure was significantly reduced by 5.2 mmHg (P=0.0417) to 7.9 mmHg (P=0.0023), compared with placebo, with a nonsignificant reduction in diastolic blood pressure.29 In another 26-week study of liraglutide in combination with metformin and rosiglitazone, the systolic blood pressure was significantly (P<0.05) reduced in the liraglutide-treated groups (1.2 mg, −6.7 mmHg; 1.8 mg, −5.6 mmHg), compared with placebo (−1.1 mmHg), with no change in diastolic blood pressure.26
The effects of the GLP-1 agonists on serum lipids are either neutral or beneficial with small, nonsignificant decreases in low-density lipoprotein (LDL-) cholesterol, increases in high-density lipoprotein (HDL-) cholesterol, and occasionally, significant decreases in fasting triglyceride levels (Table 3).30
The improvement in weight reduction with the use of GLP-1 agonists appears to be superior to their overall ability to affect marked and consistent changes in lipid levels. Most importantly, the GLP-1 agonists have a significant effect on reducing lipemia. Mechanisms by which GLP-1 agonists tend to improve postprandial lipids would include their ability to delay gastric emptying,9 and to reinforce the ability of insulin to inhibit very low density lipoprotein-triglyceride production.31
Safety
In the 30-week placebo-controlled clinical trials of exenatide, the most frequent AEs were nausea, hypoglycemia, vomiting, diarrhea, dizziness, headache, and dyspepsia.18 Nausea is usually mild or moderate, and decreases over time.18 Hypoglycemia is rare. Exenatide therapy should not be prescribed for patients with severe renal impairment or end-stage renal disease, and initiation of exenatide b.i.d. or dose escalation should be done cautiously in patients with moderate renal failure.18 Exenatide b.i.d. is not recommended for patients with severe gastrointestinal disease, such as gastroparesis.18 Exenatide b.i.d. should be discontinued if a patient experiences a hypersensitivity reaction or develops pancreatitis.18
The most common adverse reactions reported in ≥5% of patients treated with liraglutide, and reported more commonly than in patients treated with placebo, include headache, nausea, diarrhea, and anti-liraglutide antibody formation.12 Clinical trials have also shown that immunogenicity-related events, including urticaria and angioedema, were more common among liraglutide-treated patients (0.8%) than among comparator-treated patients (0.4%).12 Transient nausea is usually mild to moderate and decreases over time, so that by week 26 of the LEAD-6 study, 9% of exenatide-treated patients and 3% of liraglutide-treated patients continued to experience nausea.15 Hypoglycemia is rare. There is no dosage adjustment needed for patients with renal impairment.12
Patients with a personal or family history of medullary thyroid carcinoma (MTC) should not use liraglutide.12 MTC is an extremely rare form of thyroid cancer, with a prevalence rate of less than 600 new cases per year in the United States.32 In preclinical studies, liraglutide given to rodents in doses many times greater than the maximal doses anticipated in humans increased the incidence of C-cell tumors.32 Similar increases in clinical markers and the development of C-cell tumors have been seen in rodent studies involving other GLP-1 agonists, including exenatide, taspoglutide, and lixsenatide.6 In human volunteer studies, no link between liraglutide and C-cell tumors has ever been identified, and the United States Food and Drug Administration (FDA) determined that the risk of thyroid cancer among humans treated with liraglutide was low.32 GLP-1 receptor activation and expression appears to be species-specific;6 20 months of liraglutide treatment at 60 times the normal human exposure levels failed to induce C-cell pathology in monkeys.6 Mean calcitonin levels in patients exposed to liraglutide for 2 years also remained at the lower end of normal, when compared with placebo and comparator drugs in the clinical trials.6 Elevated levels of calcitonin (>20 pg/mL) can be indicative of C-cell pathology in humans.6 However, the FDA has indicated that monitoring calcitonin levels in liraglutide-treated patients is not recommended.32
Conclusion
GLP-1 receptor agonists have been developed to address the direct pathophysiologic defects observed in T2DM. Because GLP-1 receptor agonists work in a glucose-dependent manner, they are likely to reduce hyperglycemia safely, without a marked fluctuation toward hypoglycemia. In the process of acutely restoring β-cell function, GLP-1 agonists may allow patients to achieve HbA1c <7%, without experiencing weight gain or hypoglycemia.
The safety and efficacy of this class of medications appears to be promising. Although no medication has been shown to be effective at reducing cardiovascular events, GLP-1 agonists are being studied in long-term clinical trials to determine whether they may play a role in reducing long-term cardiovascular mortality. The ability of GLP-1 receptor agonists to improve blood pressure and postprandial lipidemia in the context of weight neutrality or weight loss may have the potential to ameliorate some of the cardiovascular risks observed in patients with T2DM.
References
Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076–1082.
Nauck MA, Busing M, Orskov C, et al. Preserved incretin effect in type 1 diabetic patients with end-stage nephropathy treated by combined heterotopic pancreas and kidney transplantation. Acta Diabetol. 1993;30:39–45.
Holst JJ, Vilsboll T, Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol. 2009;297:127–136.
Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–3723.
Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165.
Knudsen LB, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473–1486.
Deacon CF, Nauck MA, Meier J, Hucking K, Holst JJ. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab. 2000;85:3575–3581.
Pratley RE, Gilbert M. Targeting incretins in type 2 diabetes: role of GLP-1 receptor agonists and DPP-4 inhibitors. Rev Diabet Stud. 2008;5:73–94.
Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24:2943–2952.
Christensen M, Knop FK. Once-weekly GLP-1 agonists: how do they differ from exenatide and liraglutide? Curr Diab Rep. 2010;10:124–132.
Fineman MS, Bicsak TA, Shen LZ, et al. Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care. 2003;26:2370–2377.
Victoza [package insert]. Princeton, NJ: Novo Nordisk, Inc. January 20, 2010.
Steensgaard DB, Thomsen JK, Olsen HB, Knudsen SM. The molecular basis for the delayed absorption of the once-daily human GLP-1 analogue, liraglutide [abstract]. Diabetes. 2008;57(suppl. 1):A164. Abstract 552-P.
Agerso H, Jensen LB, Elbrond B, Rolan P, Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45:195–202.
Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39–47.
Buse JB, Sesti G, Schmidt WE, et al. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010;33:1300–1303.
Glucophage [package insert]. Princeton, NJ: Bristol-Myers Squibb Company. January 1, 2009.
Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc. January 12, 2007.
Unger J. Incretins: clinical perspectives, relevance, and applications for the primary care physician in the treatment of patients with type 2 diabetes. Mellitus Mayo Clinic Proceedings. 2010;85:S38–S49.
Buse J, Montanya D, Sesti G, et al. Frequency and magnitude of antibody formation are lower with liraglutide than exenatide: LEAD-6 results. Paper presented at: 70th Scientific Sessions of the American Diabetes Association; June 25–29, 2010; Orlando, FL. Abstract 0676-P.
Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–2659.
Fehse F, Trautmann M, Holst JJ, et al. Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:5991–5997.
Chang AM, Jakobsen G, Sturis J, et al. The GLP-1 derivative NN2211 restores beta-cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes. 2003;52:1786–1791.
Vilsboll T, Brock B, Perrild H, et al. Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with type 2 diabetes mellitus. Diabet Med. 2008;25:152–156.
Bunck MC, Diamant M, Cornér A, et al. Oneyear treatment with exenatide improves betacell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009;32:762–768.
Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care. 2009;32:1224–1230.
Mafong DD, Henry RR. The role of incretins in cardiovascular control. Curr Hypertens Rep. 2009;11:18–22.
Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275–286.
Vilsboll T, Zdravkovic M, Le-Thi T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care. 2007;30:1608–1610.
Plutzky J, Garber A, Falahati A, Taft AD, Paultzer NR. Reductions in lipids and CV risk markers in patients with type 2 diabetes treated with liraglutide: a meta-analysis. Paper presented at: 20th World Diabetes Congress; October 18–22, 2009; Montreal, Canada. Abstract O-0542. Available at: www.diabetes.ca. Accessed August 15, 2010.
Parlevliet ET, de Leeuw van Weenen JE, Romijn JA, Pijl H. GLP-1 treatment reduces endogenous insulin resistance via activation of central GLP-1 receptors in mice fed a high-fat diet. Am J Physiol Endocrinol Metab. 2010;299:E318–E324.
Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide—the FDA’s review of a new antidiabetic therapy. N Engl J Med. 2010;362:774–777.
Acknowledgments
We acknowledge and thank Novo Nordisk Inc. for funding the development of this manuscript through a grant.
Jeffrey R. Unger, MD, has served as an advisory board member for Amylin Pharmaceuticals, Inc., and Novo Nordisk Pharmaceuticals, Inc. He has received honoraria as a member of the speakers bureau for Eli Lilly and Company, Novo Nordisk Pharmaceuticals, Inc., Amylin Pharmaceuticals, Inc., and has received research grants from Amylin Pharmaceuticals, Inc., Takeda Pharmaceuticals North America, Inc., Novo Nordisk Pharmaceuticals, Inc., Primary Care Education Consortium, Roche USA, GSK Pharmaceuticals, and Sanofi-aventis.
Christopher G. Parkin, MS, has received consulting fees and funding for educational programs from Abbott Diabetes Care, Amylin Pharmaceuticals, Inc., Bayer Diagnostics, Eli Lilly and Company, Generex Biotechnology Corp., LifeScan, Inc., Medtronic MiniMed, Inc., Primary Care Education Consortium, Roche Diagnostics Corp., Sanofi-aventis, and Smiths Medical.
Christopher G. Parkin is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Open Access. This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Unger, J.R., Parkin, C.G. Glucagon-like peptide-1 (GLP-1) receptor agonists: Differentiating the new medications. Diab Ther 2, 29–39 (2011). https://doi.org/10.1007/s13300-010-0013-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-010-0013-5