Abstract
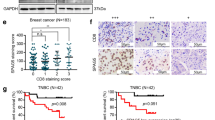
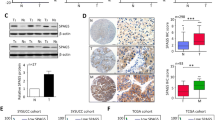
Recently, we demonstrated the association of sperm-associated antigen 9 (SPAG9) expression with breast cancer. Among breast cancer, 15 % of the cancers are diagnosed as triple-negative breast cancers (TNBC) based on hormone receptor status and represent an important clinical challenge because of lack of effective available targeted therapy. Therefore, in the present investigation, plasmid-based small hairpin (small hairpin RNA (shRNA)) approach was used to ablate SPAG9 in aggressive breast cancer cell line model (MDA-MB-231) in order to understand the role of SPAG9 at molecular level in apoptosis, cell cycle, and epithelial-to-mesenchymal transition (EMT) signaling. Our data in MDA-MB-231 cells showed that ablation of SPAG9 resulted in membrane blebbing, increased mitochondrial membrane potential, DNA fragmentation, phosphatidyl serine surface expression, and caspase activation. SPAG9 depletion also resulted in cell cycle arrest in G0–G1 phase and induced cellular senescence. In addition, in in vitro and in vivo xenograft studies, ablation of SPAG9 resulted in upregulation of p21 along with pro-apoptotic molecules such as BAK, BAX, BIM, BID, NOXA, AIF, Cyto-C, PARP1, APAF1, Caspase 3, and Caspase 9 and epithelial marker, E-cadherin. Also, SPAG9-depleted cells showed downregulation of cyclin B1, cyclin D1, cyclin E, CDK1, CDK4, CDK6, BCL2, Bcl-xL, XIAP, cIAP2, MCL1, GRP78, SLUG, SNAIL, TWIST, vimentin, N-cadherin, MMP2, MMP3, MMP9, SMA, and β-catenin. Collectively, our data suggests that SPAG9 promotes tumor growth by inhibiting apoptosis, altering cell cycle, and enhancing EMT signaling in in vitro cells and in vivo mouse model. Hence, SPAG9 may be a potential novel target for therapeutic use in TNBC treatment.





Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29.
Porter P. “Westernizing” women’s risks? Breast cancer in lower-income countries. N Engl J Med. 2008;358:213–6.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48.
Suri A, Saini S, Sinha A, Agarwal S, Verma A, Parashar D, et al. Cancer testis antigens: a new paradigm for cancer therapy. Oncoimmunology. 2012;1:1194–6.
Kanojia D, Garg M, Gupta S, Gupta A, Suri A. Sperm-associated antigen 9, a novel biomarker for early detection of breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:630–9.
Sinha A, Agarwal S, Parashar D, Verma A, Saini S, Jagadish N, et al. Down regulation of SPAG9 reduces growth and invasive potential of triple-negative breast cancer cells: possible implications in targeted therapy. J Exp Clin Cancer Res. 2013;32:69.
Jagadish N, Parashar D, Gupta N, Agarwal S, Purohit S, Kumar V, et al. A-kinase anchor protein 4 (AKAP4) a promising therapeutic target of colorectal cancer. J Exp Clin Cancer Res. 2015;34:142.
Rana R, Jagadish N, Garg M, Mishra D, Dahiya N, Chaurasiya D, et al. Small interference RNA-mediated knockdown of sperm associated antigen 9 having structural homology with c-Jun N-terminal kinase interacting protein. Biochem Biophys Res Commun. 2006;340:158–64.
Simpson AJG, Caballero OL, Jungbluth A, Chen Y-T, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–25.
Fratta E, Coral S, Covre A, Parisi G, Colizzi F, Danielli R, et al. The biology of cancer testis antigens: putative function, regulation and therapeutic potential. Mol Oncol. 2011;5:164–82.
Ademuyiwa FO, Bshara W, Attwood K, Morrison C, Edge SB, Karpf AR, et al. NY-ESO-1 cancer testis antigen demonstrates high immunogenicity in triple negative breast cancer. PLoS One. 2012;7:e38783.
Badovinac Črnjević T, Tanja BČ, Spagnoli G, Giulio S, Juretić A, Antonio J, et al. High expression of MAGE-A10 cancer-testis antigen in triple-negative breast cancer. Med Oncol. 2012;29:1586–91.
Curigliano G, Viale G, Ghioni M, Jungbluth AA, Bagnardi V, Spagnoli GC, et al. Cancer-testis antigen expression in triple-negative breast cancer. Ann Oncol. 2011;22:98–103.
Deshpande A, Sicinski P, Hinds PW. Cyclins and cdks in development and cancer: a perspective. Oncogene. 2005;24:2909–15.
Por E, Byun HJ, Lee EJ, Lim JH, Jung SY, Park I, et al. The cancer/testis antigen CAGE with oncogenic potential stimulates cell proliferation by up-regulating cyclins D1 and E in an AP-1- and E2F-dependent manner. J Biol Chem. 2010;285:14475–85.
Deng Q, Li KY, Chen H, Dai JH, Zhai YY, Wang Q, et al. RNA interference against cancer/testis genes identifies dual specificity phosphatase 21 as a potential therapeutic target in human hepatocellular carcinoma. Hepatology. 2014;59:518–30.
Alberti L, Renaud S, Losi L, Leyvraz S, Benhattar J. High expression of hTERT and stemness genes in BORIS/CTCFL positive cells isolated from embryonic cancer cells. PLoS One. 2014;9:e109921.
Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011;29:4828–36.
Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32.
Zhou B, Li T, Liu Y, Zhu N. Preliminary study on XAGE-1b gene and its mechanism for promoting tumor cell growth. Biomed Reports. 2013;1:567–72.
Ladelfa MF, Peche LY, Toledo MF, Laiseca JE, Schneider C, Monte M. Tumor-specific MAGE proteins as regulators of p53 function. Cancer Lett. 2012;325:11–7.
Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009.
Spaderna S, Schmalhofer O, Wahlbuhl M, Dimmler A, Bauer K, Sultan A, et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008;68:537–44.
Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39.
Yang P. Cancer/testis antigens trigger epithelial-mesenchymal transition and genesis of cancer stem-like cells. Curr Pharm Des. 2015;21:1292–300.
Cronwright G, Le Blanc K, Götherström C, Darcy P, Ehnman M, Brodin B. Cancer/testis antigen expression in human mesenchymal stem cells: down-regulation of SSX impairs cell migration and matrix metalloproteinase 2 expression. Cancer Res. 2005;65:2207–15.
Acknowledgments
This work is supported by grants from Indo-UK Cancer Research Program (Grant No. BT/IN/UK/NII/2006), Centre for Molecular Medicine (Grant No. BT/PR/14549/MED/14/1291), NII-core funding, Department of Biotechnology, Government of India. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. We acknowledge Dr V. Kumar, Senior Staff Scientist, International Centre for Genetic Engineering and Biotechnology, New Delhi, India, for critical reading and editing of this manuscript. We also thank technical support by Mrs. Rekha Rani, National Institute of Immunology, New Delhi, India, for SEM imaging.
Authors’ contributions
NJ, NG, SA, APT, DP, RF, AS, and VK carried out all the experiments, prepared the figures, and drafted the manuscript. NJ participated in data analysis and interpretation of results. AS designed the study and participated in data analysis and interpretation of results. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Namita Gupta and Sumit Agarwal contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Western blot analysis demonstrates up-regulation of apoptotic moleclues, BAD, BAX, cyto-C, Caspase 7 and down-regulation of anti-apoptotic molecule, BCL-XL in SPAG9 ablated MCF7, BT-474 and SK-BR-3 breast cancer cells. β-actin was used as a loading control. Western Blotting was repeated in three independent experiments. (PPTX 805 kb)
Rights and permissions
About this article
Cite this article
Jagadish, N., Gupta, N., Agarwal, S. et al. Sperm-associated antigen 9 (SPAG9) promotes the survival and tumor growth of triple-negative breast cancer cells. Tumor Biol. 37, 13101–13110 (2016). https://doi.org/10.1007/s13277-016-5240-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5240-6