Abstract
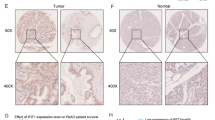
Chromosome 1p35-36, which encodes tumor suppressors and mitotic checkpoint control genes, is commonly altered in human malignancies. One gene at this locus, stathmin 1 (STMN1), is involved in cell cycle progression and metastasis. We hypothesized that increased STMN1 expression may play a role in pancreatic neuroendocrine neoplasm (pNEN) malignancy. We investigated stathmin copy number variation, mRNA, and protein expression using PCR-Taqman Copy Number Assays, Q-PCR, Western blot, and immunohistochemistry. A mechanistic role for stathmin in proliferation was assessed in the BON cell line under growth-restrictive conditions and siRNA silencing. Furthermore, its role in PI3K signaling pathway activation was evaluated using pharmacological inhibitors. mRNA (p = 0.0001) and protein (p < 0.05) were overexpressed in pNENs. Expression was associated with pNEN tumor extension (p < 0.05), size (p < 0.01), and Ki67 expression (p < 0.01). Serum depletion decreased Ki67 expression (p < 0.01) as well as Ser38 phosphorylation (p < 0.05) in BON cells. STMN1 knockdown (siRNA) decreased proliferation (p < 0.05), and PI3K inhibitors directly inhibited proliferation via stathmin inactivation (dephosphorylation p < 0.01). We identified that stathmin was overexpressed and associated with pathological parameters in pancreatic NENs. We postulate that STMN1 overexpression and phosphorylation result in a loss of cell cycle mitotic checkpoint control and may render tumors amenable to PI3K inhibitory therapy.




Similar content being viewed by others
References
Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72.
Schimmack S, Svejda B, Lawrence B, Kidd M, Modlin IM. The diversity and commonalities of gastroenteropancreatic neuroendocrine tumors. Langenbecks Arch Surg. 2011;396:273–98.
Canavese G, Azzoni C, Pizzi S, Corleto VD, Pasquali C, Davoli C, et al. p27: a potential main inhibitor of cell proliferation in digestive endocrine tumors but not a marker of benign behavior. Hum Pathol. 2001;32:1094–101.
Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–203.
Raef H, Zou M, Baitei EY, Al-Rijjal RA, Kaya N, Al-Hamed M, et al. A novel deletion of the MEN1 gene in a large family of multiple endocrine neoplasia type 1 (MEN1) with aggressive phenotype. Clin Endocrinol (Oxf). 2011;75:791–800.
Hu W, Feng Z, Modica I, Klimstra DS, Song L, Allen PJ, et al. Gene amplifications in well-differentiated pancreatic neuroendocrine tumors inactivate the p53 pathway. Genes Cancer. 2010;1:360–8.
Tang LH, Contractor T, Clausen R, Klimstra DS, Du YC, Allen PJ, et al. Attenuation of the retinoblastoma pathway in pancreatic neuroendocrine tumors due to increased cdk4/cdk6. Clin Cancer Res. 2012;18:4612–20.
Zikusoka MN, Kidd M, Eick G, Latich I, Modlin IM. The molecular genetics of gastroenteropancreatic neuroendocrine tumors. Cancer. 2005;104:2292–309.
Zhao J, Moch H, Scheidweiler AF, Baer A, Schaffer AA, Speel EJ, et al. Genomic imbalances in the progression of endocrine pancreatic tumors. Genes Chromosomes Cancer. 2001;32:364–72.
Beckers A, Abs R, Reyniers E, De Boulle K, Stevenaert A, Heller FR, et al. Variable regions of chromosome 11 loss in different pathological tissues of a patient with the multiple endocrine neoplasia type I syndrome. J Clin Endocrinol Metab. 1994;79:1498–502.
Janoueix-Lerosey I, Novikov E, Monteiro M, Gruel N, Schleiermacher G, Loriod B, et al. Gene expression profiling of 1p35-36 genes in neuroblastoma. Oncogene. 2004;23:5912–22.
Ezaki T, Yanagisawa A, Ohta K, Aiso S, Watanabe M, Hibi T, et al. Deletion mapping on chromosome 1p in well-differentiated gastric cancer. Br J Cancer. 1996;73:424–8.
Leister I, Weith A, Bruderlein S, Cziepluch C, Kangwanpong D, Schlag P, et al. Human colorectal cancer: high frequency of deletions at chromosome 1p35. Cancer Res. 1990;50:7232–5.
Hahn SA, Seymour AB, Hoque AT, Schutte M, da Costa LT, Redston MS, et al. Allelotype of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res. 1995;55:4670–5.
Ebrahimi SA, Wang EH, Wu A, Schreck RR, Passaro Jr E, Sawicki MP. Deletion of chromosome 1 predicts prognosis in pancreatic endocrine tumors. Cancer Res. 1999;59:311–5.
Stumpf E, Aalto Y, Hoog A, Kjellman M, Otonkoski T, Knuutila S, et al. Chromosomal alterations in human pancreatic endocrine tumors. Genes Chromosomes Cancer. 2000;29:83–7.
Rubin CI, Atweh GF. The role of stathmin in the regulation of the cell cycle. J Cell Biochem. 2004;93:242–50.
Sherbet G, Cajone F. Stathmin in cell proliferation and cancer progression. Cancer Genomics Proteomics. 2005;2:227–38.
Jourdain L, Curmi P, Sobel A, Pantaloni D, Carlier MF. Stathmin: a tubulin-sequestering protein which forms a ternary T2S complex with two tubulin molecules. Biochemistry. 1997;36:10817–21.
Lin X, Tang M, Tao Y, Li L, Liu S, Guo L, et al. Epstein-Barr virus-encoded LMP1 triggers regulation of the ERK-mediated Op18/stathmin signaling pathway in association with cell cycle. Cancer Sci. 2012;103:993–9.
Tan HT, Wu W, Ng YZ, Zhang X, Yan B, Ong CW, et al. Proteomic analysis of colorectal cancer metastasis: stathmin-1 revealed as a player in cancer cell migration and prognostic marker. J Proteome Res. 2012;11:1433–45.
Jeon TY, Han ME, Lee YW, Lee YS, Kim GH, Song GA, et al. Overexpression of stathmin1 in the diffuse type of gastric cancer and its roles in proliferation and migration of gastric cancer cells. Br J Cancer. 2010;102:710–8.
Singer S, Malz M, Herpel E, Warth A, Bissinger M, Keith M, et al. Coordinated expression of stathmin family members by far upstream sequence element-binding protein-1 increases motility in non-small cell lung cancer. Cancer Res. 2009;69:2234–43.
Hsieh SY, Huang SF, Yu MC, Yeh TS, Chen TC, Lin YJ, et al. Stathmin1 overexpression associated with polyploidy, tumor-cell invasion, early recurrence, and poor prognosis in human hepatoma. Mol Carcinog. 2010;49:476–87.
Ghosh R, Gu G, Tillman E, Yuan J, Wang Y, Fazli L, et al. Increased expression and differential phosphorylation of stathmin may promote prostate cancer progression. Prostate. 2007;67:1038–52.
Baquero MT, Hanna JA, Neumeister V, Cheng H, Molinaro AM, Harris LN, et al. Stathmin expression and its relationship to microtubule-associated protein tau and outcome in breast cancer. Cancer. 2012.
Wei SH, Lin F, Wang X, Gao P, Zhang HZ. Prognostic significance of stathmin expression in correlation with metastasis and clinicopathological characteristics in human ovarian carcinoma. Acta Histochem. 2008;110:59–65.
Xi W, Rui W, Fang L, Ke D, Ping G, Hui-Zhong Z. Expression of stathmin/op18 as a significant prognostic factor for cervical carcinoma patients. J Cancer Res Clin Oncol. 2009;135:837–46.
Trovik J, Wik E, Stefansson IM, Marcickiewicz J, Tingulstad S, Staff AC, et al. Stathmin overexpression identifies high-risk patients and lymph node metastasis in endometrial cancer. Clin Cancer Res. 2011;17:3368–77.
Hanash SM, Strahler JR, Kuick R, Chu EH, Nichols D. Identification of a polypeptide associated with the malignant phenotype in acute leukemia. J Biol Chem. 1988;263:12813–5.
Kidd M, Eick G, Shapiro MD, Camp RL, Mane SM, Modlin IM. Microsatellite instability and gene mutations in transforming growth factor-beta type II receptor are absent in small bowel carcinoid tumors. Cancer. 2005;103:229–36.
Lopez JR, Claessen SM, Macville MV, Albrechts JC, Skogseid B, Speel EJ. Spectral karyotypic and comparative genomic analysis of the endocrine pancreatic tumor cell line BON-1. Neuroendocrinology. 2010;91:131–41.
Sirivatanauksorn V, Sirivatanauksorn Y, Gorman PA, Davidson JM, Sheer D, Moore PS, et al. Non-random chromosomal rearrangements in pancreatic cancer cell lines identified by spectral karyotyping. Int J Cancer. 2001;91:350–8.
Schimmack S, Lawrence B, Svejda B, Alaimo D, Schmitz-Winnenthal H, Fischer L, et al. The clinical implications and biologic relevance of neurofilament expression in gastroenteropancreatic neuroendocrine neoplasms. Cancer. 2012;118:2763–75.
Svejda B, Kidd M, Kazberouk A, Lawrence B, Pfragner R, Modlin IM. Limitations in small intestinal neuroendocrine tumor therapy by mTOR kinase inhibition reflect growth factor-mediated PI3K feedback loop activation via ERK1/2 and AKT. Cancer. 2011;117:4141–54.
Kidd M, Nadler B, Mane S, Eick G, Malfertheiner M, Champaneria M, et al. GeneChip, geNorm, and gastrointestinal tumors: novel reference genes for real-time PCR. Physiol Genomics. 2007;30:363–70.
Tan HT, Wu W, Ng YZ, Zhang X, Yan B, Ong CW, et al. Proteomic analysis of colorectal cancer metastasis: stathmin-1 revealed as a player in cancer cell migration and prognostic marker. J Proteome Res. 2012;10:10.
Evers BM, Townsend Jr CM, Upp JR, Allen E, Hurlbut SC, Kim SW, et al. Establishment and characterization of a human carcinoid in nude mice and effect of various agents on tumor growth. Gastroenterology. 1991;101:303–11.
Modlin I, Moss SF, Gustafsson BI, Lawrence B, Schimmack S, Kidd M. The archaic distinction between functioning and non-functioning neuroendocrine neoplasms is no longer clinically relevant. Langenbecks Arch Surg. 2011.
Andersen JN, Sathyanarayanan S, Di Bacco A, Chi A, Zhang T, Chen AH, et al. Pathway-based identification of biomarkers for targeted therapeutics: personalized oncology with PI3K pathway inhibitors. Sci Transl Med. 2010;2:43ra55.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Lubensky IA, Zhuang Z. Molecular genetic events in gastrointestinal and pancreatic neuroendocrine tumors. Endocr Pathol. 2007;18:156–62.
Mathew CG, Smith BA, Thorpe K, Wong Z, Royle NJ, Jeffreys AJ, et al. Deletion of genes on chromosome 1 in endocrine neoplasia. Nature. 1987;328:524–6.
Zhang HZ, Wang Y, Gao P, Lin F, Liu L, Yu B, et al. Silencing stathmin gene expression by survivin promoter-driven siRNA vector to reverse malignant phenotype of tumor cells. Cancer Biol Ther. 2006;5:1457–61.
Belletti B, Nicoloso MS, Schiappacassi M, Berton S, Lovat F, Wolf K, et al. Stathmin activity influences sarcoma cell shape, motility, and metastatic potential. Mol Biol Cell. 2008;19:2003–13.
Baldassarre G, Belletti B, Nicoloso MS, Schiappacassi M, Vecchione A, Spessotto P, et al. p27 (Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell. 2005;7:51–63.
Rowlands DC, Harrison RF, Jones NA, Williams A, Hubscher SG, Brown G. Stathmin is expressed by the proliferating hepatocytes during liver regeneration. Clin Mol Pathol. 1995;48:M88–92.
Sadow PM, Rumilla KM, Erickson LA, Lloyd RV. Stathmin expression in pheochromocytomas, paragangliomas, and in other endocrine tumors. Endocr Pathol. 2008;19:97–103.
Wik E, Birkeland E, Trovik J, Werner HM, Hoivik EA, Mjos S, et al. High phospho-stathmin (Serine38) expression identifies aggressive endometrial cancer and suggests an association with PI3Kinase inhibition. Clin Cancer Res. 2013;28:28.
Parker CG, Hunt J, Diener K, McGinley M, Soriano B, Keesler GA, et al. Identification of stathmin as a novel substrate for p38 delta. Biochem Biophys Res Commun. 1998;249:791–6.
Salvesen HB, Carter SL, Mannelqvist M, Dutt A, Getz G, Stefansson IM, et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci U S A. 2009;106:4834–9.
Saal LH, Johansson P, Holm K, Gruvberger-Saal SK, She QB, Maurer M, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci U S A. 2007;104:7564–9. Epub 2007 Apr 7523.
Brattsand G, Marklund U, Nylander K, Roos G, Gullberg M. Cell-cycle-regulated phosphorylation of oncoprotein 18 on Ser16, Ser25 and Ser38. Eur J Biochem. 1994;220:359–68.
Larsson N, Melander H, Marklund U, Osterman O, Gullberg M. G2/M transition requires multisite phosphorylation of oncoprotein 18 by two distinct protein kinase systems. J Biol Chem. 1995;270:14175–83.
Singer S, Ehemann V, Brauckhoff A, Keith M, Vreden S, Schirmacher P, et al. Protumorigenic overexpression of stathmin/Op18 by gain-of-function mutation in p53 in human hepatocarcinogenesis. Hepatology. 2007;46:759–68.
Zheng P, Liu YX, Chen L, Liu XH, Xiao ZQ, Zhao L, et al. Stathmin, a new target of PRL-3 identified by proteomic methods, plays a key role in progression and metastasis of colorectal cancer. J Proteome Res. 2010;9:4897–905.
Liang XJ, Choi Y, Sackett DL, Park JK. Nitrosoureas inhibit the stathmin-mediated migration and invasion of malignant glioma cells. Cancer Res. 2008;68:5267–72.
Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol. 2003;5:599–609.
Nogales E, Wang HW. Structural intermediates in microtubule assembly and disassembly: how and why? Curr Opin Cell Biol. 2006;18:179–84.
Yoshie M, Miyajima E, Kyo S, Tamura K. Stathmin, a microtubule regulatory protein, is associated with hypoxia-inducible factor-1alpha levels in human endometrial and endothelial cells. Endocrinology. 2009;150:2413–8.
Saal LH, Johansson P, Holm K, Gruvberger-Saal SK, She QB, Maurer M, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci U S A. 2007;104:7564–9.
Drury SC, Detre S, Leary A, Salter J, Reis-Filho J, Barbashina V, et al. Changes in breast cancer biomarkers in the IGF1R/PI3K pathway in recurrent breast cancer after tamoxifen treatment. Endocr Relat Cancer. 2011;18:565–77.
Wang X, Ren JH, Lin F, Wei JX, Long M, Yan L, et al. Stathmin is involved in arsenic trioxide-induced apoptosis in human cervical cancer cell lines via PI3K linked signal pathway. Cancer Biol Ther. 2010;10:632–43.
Karst AM, Levanon K, Duraisamy S, Liu JF, Hirsch MS, Hecht JL, et al. Stathmin 1, a marker of PI3K pathway activation and regulator of microtubule dynamics, is expressed in early pelvic serous carcinomas. Gynecol Oncol. 2011;123:5–12.
Acknowledgments
Funding support for SS was provided by the Deutsche Forschungsgemeinschaft SCHI 1177/1-1. BL was partially supported by the Murray Jackson Clinical Fellowship from the Genesis Oncology Trust, Auckland, New Zealand.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Schimmack, S., Taylor, A., Lawrence, B. et al. Stathmin in pancreatic neuroendocrine neoplasms: a marker of proliferation and PI3K signaling. Tumor Biol. 36, 399–408 (2015). https://doi.org/10.1007/s13277-014-2629-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2629-y