Abstract
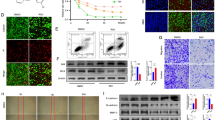
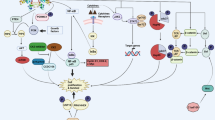
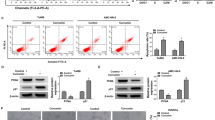
Myricetin, a common dietary flavonoid, is widely distributed in fruits and vegetables and is used as a health food supplement based on its anti-tumor properties. However, the effect and mechanisms of myricetin in esophageal carcinoma are not fully understood. Here, we demonstrated the effect of myricetin on the proliferation, apoptosis, and invasion of the esophageal carcinoma cell lines EC9706 and KYSE30 and explored the underlying mechanism and target protein(s) of myricetin. CCK-8 assay, transwell invasion assay, wound-healing assay, cell cycle analysis, and apoptosis assay were used to evaluate the effects of myricetin on cell proliferation, invasion, and apoptosis. Nude mouse tumor xenograft model was built to understand the interaction between myricetin and NTD RSK2. Pull-down assay was used to verify molecular mechanism. Myricetin inhibited proliferation and invasion and induced apoptosis of EC9706 and KYSE30 cells. Moreover, myricetin was shown to bind RSK2 through the NH2-terminal kinase domain. Finally, myricetin inhibited EC9706 and KYSE30 cell proliferation through Mad1 and induced cell apoptosis via Bad. Myricetin inhibits the proliferation and invasion and induces apoptosis in EC9706 and KYSE30 cells via RSK2. Myricetin exerts anti-proliferative, anti-invasive, and pro-apoptotic effects on esophageal carcinoma EC9706 and KYSE30 cells via RSK2. Our results provide novel insight into myricetin as a potential agent for the prevention and treatment of esophageal carcinoma.







Similar content being viewed by others
References
Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases withdiverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–44.
Jones SW, Erikson E, Blenis J, Maller JL, Erikson RL. A Xenopus ribosomal protein S6 kinase has two apparent kinase domains that are each similar to distinct protein kinases. Proc Natl Acad Sci U S A. 1988;85:3377–81.
Frodin M, Jensen CJ, Merienne K, Gammeltoft S A phosphoserine-regulated docking site in the protein kinase RSK2 that recruits and activates (2000) PDK1. EMBO J 19:2924–2934.
Artamonov M, Momotani K, Utepbergenov D, Franke A, Khromov A, Derewenda ZS. The p90 ribosomal S6 kinase (RSK) is a mediator of smooth muscle contractility. PLoS One. 2013;8(3):e58703.
Nebreda AR, Gavin AC. Perspectives: signal transduction. Cell survival demands some RSK Science. 1999;286:1309–10.
Redman EK, Brookes PS, Karcz MK. Role of p90 (RSK) in regulating the Crabtree effect: implications for cancer. Biochem. Soc Trans. 2013;41(1):124–6.
Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44.
Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol. 2005;31:151–74.
Yong HY, Koh MS, Moon A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin Investig Drugs. 2009;18(12):1893–905.
Meyuhas O. Physiological roles of ribosomal protein S6: one of its kinds. Int Rev Cell Mol Biol. 2008;268:1–37.
Cho YY, Yao K, Kim HG, Kang BS, Zheng D, Bode AM. Ribosomal S6 kinase 2 is a key regulator in tumor promoter induced cell transformation. Cancer Res. 2007;Cancer Res 67:8104–12.
Weng CJ, Yen GC. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012;31(1–2):323–51.
López-Lázaro M, Willmore E, Austin CA. The dietary flavonoids myricetin and fisetin act as dual inhibitors of DNA topoisomerases I and II in cells. Mutat Res. 2010;696(1):41–7.
Nirmala P, Ramanathan M. Effect of myricetin on 1, 2 dimethylhydrazine induced rat colon carcinogenesis. J Exp Ther Oncol. 2011;9(2):101–8.
Sun F, Zheng XY, Ye J, Wu TT, Jl W, Chen W. Potential anticancer activity of myricetin in human T24 bladder cancer cells both in vitro and in vivo. Nutr Cancer. 2012;64(4):599–606.
Jung SK, Lee KW, Byun S, Kang NJ, Lim SH, et al. Myricetin suppresses UVB-induced skin cancer by targeting Fyn. Cancer Res. 2008;68(14):6021–9.
Rodgers EH, Grant MH. The effect of the flavonoids, quercetin, myricetin and epicatechin on the growth and enzyme activities of MCF7 human breast cancer cells. Chem Biol Interact. 1998;116(3):213–28.
Kuntz S, Wenzel U, Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr. 1999;38(3):133–42.
Maggiolini M, Recchia AG, Bonofiglio D, Catalano S, Vivacqua A, Carpino A. The red wine phenolics piceatannol and myricetin act as agonists for estrogen receptor alpha in human breast cancer cells. J Mol Endocrinol. 2005.
Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9(10):747–58.
RJ S, Bates PA, Waelkens E, Dallman M, Lamb J. A siRNA screen identifies RSK1 as a key modulator of lung cancer metastasis. Oncogene. 2011;30:3513–21.
Gawecka JE, Young-Robbins SS, Sulzmaier FJ, Caliva MJ, Heikkilä MM, Matter ML. RSK2 protein suppresses integrin activation and fibronectin matrix assembly and promotes cell migration. J Biol Chem. 2012;287(52):43424–37.
van Jaarsveld MT, Blijdorp IC, Boersma AW, Pothof J, Mathijssen RH, Verweij J. The kinase RSK2 modulates the sensitivity of ovarian cancer cells to cisplatin. Eur J Cancer. 2013;49(2):345–51.
Eisinger-Mathason TS, Andrade J, Lannigan DA. RSK in tumorigenesis: connections to steroid signaling. Steroids. 2010;75(3):191–202.
Hilinski MK, Mrozowski RM, Clark DE, Lannigan DA. Analogs of the RSK inhibitor SL0101: optimization of in vitro biological stability. Bioorg Med Chem Lett. 2012;22(9):3244–7.
Lu W, Liu X, Cao X, Xue M, Liu K, Zhao Z. hybrid approach for 3D molecular similarity calculation. 2. Prospective case study in the discovery of diverse p90 ribosomal S6 protein kinase 2 inhibitors to suppress cell migration. J Med Chem. 2011;54:3564–74.
Peng C, Zhu F, Wen W, Yao K, Li S, Zykova T. Tumor necrosis factor receptor-associated factor family protein 2 is a key mediator of the epidermal growth factor-induced ribosomal S6 kinase 2/cAMP-responsive element-binding protein/Fos protein signaling pathway. J Biol Chem. 2012;287(31):25881–92.
Malakhova M, Kurinov I, Liu K, Zheng D, D'Angelo I, Shim JH. Structural diversity of the active N-terminal kinase domain of p90 ribosomal S6 kinase 2. PLoS One. 2009;4(11):e8044.
Shimura Y, Kuroda J, Ri M, Nagoshi H, Yamamoto-Sugitani M, Kobayashi T. RSK2(Ser227) at N-terminal kinase domain is a potential therapeutic target for multiple myeloma. Mol Cancer Ther. 2012;11(12):2600–9.
Utepbergenov D, Derewenda U, Olekhnovich N, Szukalska G, Banerjee B, et al. Insights into the inhibition of the p90 ribosomal S6 kinase (RSK) by the flavonol glycoside SL010 from the 1.5 Å crystal structure of the N-terminal domain of RSK2 with bound inhibitor. Biochemistry. 2012;51(33):6499–510.
Rottmann S, Lüscher B. The Mad side of the Max network: antagonizing the function of Myc and more. Curr Top Microbiol Immunol. 2006;302:63–122.
Vigneron S, Brioudes E, Burgess A, Labbé JC, Lorca T, Castro A. RSK2 is a kinetochore-associated protein that participates in the spindle assembly checkpoint. Oncogene. 2010;29(24):3566–74.
Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286(5443):1358–62.
Virdee KS, Parone PA, Tolkovsky AM. Phosphorylation of the pro-apoptotic protein BAD on serine 155, a novel site, contribute to cell survival. Curr Biol. 2000;10:1151–4.
Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286(5443):1358–62.
Conflict of interest
The authors have declared that no competing interest exists.
Acknowledgments
This study was supported by the Education Department of Henan province science and technology research projects (12A310015, 13A310671). Thank Prof. Xuejun Xu for the help in the myricetin and RSK2 interaction assay.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zang, W., Wang, T., Wang, Y. et al. Myricetin exerts anti-proliferative, anti-invasive, and pro-apoptotic effects on esophageal carcinoma EC9706 and KYSE30 cells via RSK2. Tumor Biol. 35, 12583–12592 (2014). https://doi.org/10.1007/s13277-014-2579-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2579-4