Abstract
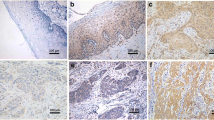
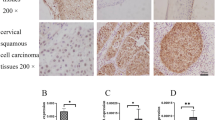
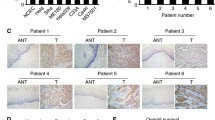
This study aimed to investigate the expression of Six1 gene and its downstream target gene Ezrin in cervical cancer, and to correlate their expression to the clinical pathology of cervical cancer. RT–PCR and Western blot were used to detect the expression of Six1 and Ezrin in cervical cancer tissue, cervical intraepithelial neoplasia tissue, and normal cervical tissue. The correlation of Six1 and Ezrin expression with the occurrence, development, and clinical pathology of cervical cancer was then analyzed. The expression of Six1 and Ezrin mRNA and protein in cervical cancer and cervical intraepithelial neoplasia was significantly higher than in normal cervical tissue (p < 0.05). Their expression was also significantly higher in the cancerous tissues of patients with advanced cervical cancer than in those of patients with early-stage cervical cancer (p < 0.05). Moreover, they were expressed at significantly higher levels in lymph node metastasis-positive patients than in lymph node metastasis-negative patients (p < 0.05). Finally, we observed that lesser differentiated tumors had elevated expression of Six1 and Ezrin mRNA and protein (p < 0.05). However, the mRNA and protein expression of Six1 and Ezrin were independent of patient age and tumor size (p > 0.05). Further investigation is warranted to describe the relation of whether Six1 and Ezrin protein contributes to the mechanism of occurrence, development, differentiation, and invasiveness of the tumor. These markers could be used as indicators of prognosis in early-stage cervical cancer as Six1 and Ezrin had a synergistic effect on the incidence of cervical cancer.


Similar content being viewed by others
References
Berger MF, Badis G, Gehrke AR, Talukder S, Philippakis AA, Pena-Castillo L, et al. Variation in homeodomain DNA binding revealed by high-resolution analysis of sequence preferences. Cell. 2008;133(7):1266–76.
Bricaud O, Collazo A. The transcription factor six1 inhibits neuronal and promotes hair cell fate in the developing zebrafish (Danio rerio) inner ear. J Neurosci. 2006;26(41):10438–51.
Christensen KL, Patrick AN, McCoy EL, Ford HL. The six family of homeobox genes in development and cancer. Adv Cancer Res. 2008;101:93–126.
Laclef C, Hamard G, Demignon J, Souil E, Houbron C, Maire P. Altered myogenesis in Six1-deficient mice. Development. 2003;130(10):2239–52.
Yu Y, Davicioni E, Triche TJ, Merlino G. The homeoprotein six1 transcriptionally activates multiple protumorigenic genes but requires ezrin to promote metastasis. Cancer Res. 2006;66(4):1982–9.
Reichenberger KJ, Coletta RD, Schulte AP, Varella-Garcia M, Ford HL. Gene amplification is a mechanism of Six1 overexpression in breast cancer. Cancer Res. 2005;65(7):2668–75.
Li Q, Wu MF, Song AP, Wei JC, Xu G, Lu YP, et al. Expression of Ezrin and E-cadherin in invasive ductal breast cancer and their correlations to lymphatic metastasis. Ai Zheng. 2006;25(3):363–6.
Benedet JL, Bender H, Jones 3rd H, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000;70(2):209–62.
Xiao DK, He JX. Epithelial mesenchymal transition and lung cancer. J Thorac Dis. 2010;2(3):154–159.
Pearson JC, Lemons D, McGinnis W. Modulating Hox gene functions during animal body patterning. Nat Rev Genet. 2005;6(12):893–904.
Kawakami K, Sato S, Ozaki H, Ikeda K. Six family genes—structure and function as transcription factors and their roles in development. Bioessays. 2000;22(7):616–26.
Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, Merlino G. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med. 2004;10(2):175–81.
Ng KT, Man K, Sun CK, Lee TK, Poon RT, Lo CM, et al. Clinicopathological significance of homeoprotein Six1 in hepatocellular carcinoma. Br J Cancer. 2006;95(8):1050–5.
Giordani J, Bajard L, Demignon J, Daubas P, Buckingham M, Maire P. Six proteins regulate the activation of Myf5 expression in embryonic mouse limbs. Proc Natl Acad Sci USA. 2007;104(27):11310–5.
Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. The FERM domain: organizing the structure and function of FAK. Nat Rev Mol Cell Biol. 2010;11(11):802–14.
Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11(4):276–87.
Granes F, Urena JM, Rocamora N, Vilaro S. Ezrin links syndecan-2 to the cytoskeleton. J Cell Sci. 2000;113(Pt 7):1267–76.
Zhang Y, Hu MY, Wu WZ, Wang ZJ, Zhou K, Zha XL, et al. The membrane-cytoskeleton organizer ezrin is necessary for hepatocellular carcinoma cell growth and invasiveness. J Cancer Res Clin Oncol. 2006;132(11):685–97.
Kang YK, Hong SW, Lee H, Kim WH. Prognostic implications of ezrin expression in human hepatocellular carcinoma. Mol Carcinog. 2010;49(9):798–804.
Elliott BE, Meens JA, SenGupta SK, Louvard D, Arpin M. The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast Cancer Res. 2005;7(3):R365–73.
Weng WH, Ahlen J, Astrom K, Lui WO, Larsson C. Prognostic impact of immunohistochemical expression of ezrin in highly malignant soft tissue sarcomas. Clin Cancer Res. 2005;11(17):6198–204.
Martin TA, Harrison G, Mansel RE, Jiang WG. The role of the CD44/ezrin complex in cancer metastasis. Crit Rev Oncol Hematol. 2003;46(2):165–86.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
Rights and permissions
About this article
Cite this article
Tan, J., Zhang, C. & Qian, J. Expression and significance of Six1 and Ezrin in cervical cancer tissue. Tumor Biol. 32, 1241–1247 (2011). https://doi.org/10.1007/s13277-011-0228-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-011-0228-8