Abstract
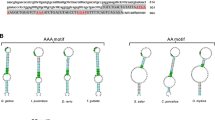
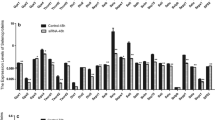
Selenoprotein M may regulate a myriad of biological processes through its redox function. In pigs, neither the nucleotide sequence nor the amino acid sequence is known. Furthermore, patterns of tissue expression and regulation by dietary selenium (Se) have not been examined. We determined the full coding sequence (CDS) and the chromosomal location of the porcine gene, SELM, and described its expression profile in vivo under different dietary Se concentrations. The cDNA sequence of porcine SELM from the start codon to the poly(A) tail was cloned by reverse transcription PCR. The CDS contained 429 bases with a typical mammalian selenocysteine insertion sequence of form 2 (F2) located in the 3′-untranslated region. The gene was mapped to chromosome 14q21, where porcine SELM and its neighboring genes exhibited a similar organization to human homologues on chromosome 22q12.2. The expression pattern of SELM mRNA in muscle, thyroid, cerebral cortex, pituitary, testis, liver, and kidney was analyzed with real-time quantitative PCR in young male pigs fed a Se-deficient corn-soybean meal basal diet supplemented with 0.0, 0.3, or 3.0 mg Se/kg in the form of Se-rich yeast. Though the SELM mRNA abundance in each of the 7 tissues was not affected by the dietary Se concentrations, it was significantly higher in thyroid (P < 0.01) than in cerebral cortex, pituitary, testis, liver, and kidney at all of the 3 dietary Se concentrations.
Similar content being viewed by others
References
Breathnach R, Chambon P (1981). Organization and expression of eukaryotic split genes coding for proteins. Ann. Rev. Biochem. 50: 349–383.
Chambers I, Frampton J, Goldfarb P, Affara N, McBain W, Harrison PR (1986) The structure of the mouse glutathione peroxidase gene: the selenocysteine in the active site is encoded by the ‘termination’ codon, TGA. EMBO J. 5: 1221–1227.
Ferguson AD, Labunskyy VM, Fomenko DE, Arac D, Chelliah Y, Amezcua CA, Rizo J, Gladyshev VN, Deisenhofer J (2006) NMR structures of the selenoproteins Sep15 and SelM reveal redox activity of a new thioredoxin-like family. J. Biol. Chem. 281: 3536–3543.
Gromer S, Eubel JK, Lee BL, Jacob J (2005) Human selenoproteins at a glance. Cell. Mol. Life. Sci. 62: 2414–2437.
Grundner-Culemann E, Martin GW 3rd, Harney JW, Berry MJ (1999) Two distinct SECIS structures capable of directing selenocysteine incorporation in eukaryotes. RNA 5: 625–635.
Hwang DY, Sin JS, Kim MS, Yim SY, Kim YK, Kim CK, Kim BG, Shim SB, Jee SW, Lee SH, Bae CJ, Lee BC, Jang MK, Cho JS, Chae KR (2008) Overexpression of human selenoprotein M differentially regulates the concentrations of antioxidants and H2O2, the activity of antioxidant enzymes, and the composition of white blood cells in a transgenic rat. Int. J. Mol. Med. 21:169–179.
Korotkov KV, Novoselov SV, Hatfield DL, Gladyshev VN (2002) Mammalian selenoprotein in which selenocysteine (Sec) incorporation is supported by a new form of Sec insertion sequence element. Mol. Cell. Biol. 22: 1402–1411.
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science. 300: 1439–1443.
Labunskyy VM, Hatfield DL, Gladyshev VN (2007) The Sep15 protein family: roles in disulfide bond formation and quality control in the endoplasmic reticulum. IUBMB Life 59: 1–5.
Lescure A, Rederstorff M, Krol A, Guicheney P, Allamand V (2009) Selenoprotein function and muscle disease. Biochim. Biophys. Acta. 1790: 1569–1574.
Mellink CH, Lahbib-Mansais Y, Yerle M, Gellin J (1993) Localization of four new markers to pig chromosomes 1, 6, and 14 by radioactive in situ hybridization. Cytogenet. Cell. Genet. 64: 256–260.
Murphy WJ (2003) The origin of human chromosome 1 and its homologs in placental mammals. Genome Res. 13: 1880–1888.
Reeves MA, Bellinger FP, Berry MJ (2010) The neuroprotective functions of selenoprotein M and its role in cytosolic calcium regulation. Antioxid. Redox. Sign. 12: 809–818.
Schomburg L, Schweizer U (2009) Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim. Biophys. Acta. 1790: 1453–1462.
Schook L, Beattie C, Beever J, Donovan S, Jamison R, Zuckermann F, Niemi S, Rothschild M, Rutherford M, Smith D (2005) Swine in biomedical research: creating the building blocks of animal models. Anim. Biotech. 16: 183–190.
Sunde RA, Raines AM, Barnes KM, Evenson JK (2009) Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci. Rep. 29: 329–338.
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24: 1596–1599.
Wassen FW, Klootwijk W, Kaptein E, Duncker DJ, Visser TJ, Kuiper GG (2004) Characteristics and thyroid state-dependent regulation of iodothyronine deiodinases in pigs. Endocrinology 145: 4251–4263.
Zambonelli P, Davoli R, Zijlstra C, Bosma AA, Russo V (1998) Assignment of gamma-glutamyltransferase (GGT) to porcine chromosome band 14q2.1 by in situ hybridization. Cytogenet. Cell. Genet. 82: 202–203.
Zhang Y, Zhou Y, Schweizer u, Savaskan NE, Hua D, Kipnis J, Hatfield DL, Gladyhev VN (2008) Comparative analysis of selenocysteine machinery and selenoproteome gene expression in mouse brain identifies neurons as key functional sites of selenium in mammals. J. Biol. Chem. 283: 2427–2438.
Zhou JC, Zhao H, Li JG, Xia XJ, Wang KN, Zhang YJ, Liu Y, Zhao Y, Lei XG (2009) Selenoprotein gene expression in thyroid and pituitary of young pigs is not affected by dietary selenium deficiency or excess. J. Nutr. 139: 1061–1066.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhou, JC., Zhao, H., Tang, JY. et al. Molecular cloning, chromosomal localization and expression profiling of porcine selenoprotein M gene. Genes Genom 33, 529–534 (2011). https://doi.org/10.1007/s13258-010-0127-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-010-0127-1