Abstract
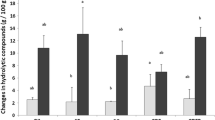
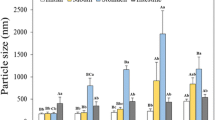
The health significance of lipid oxidation products carried by the diet or eventually formed during food digestion is actually addressed. Accordingly, some data show that the gastric environment could be prooxidant. The aim of this work was to evaluate the impact of the balance between lipophilic antioxidant species and dietary prooxidant on lipid oxidation under in vitro gastrointestinal conditions. Rapeseed oil-in-water emulsions stabilized by bovine serum albumin were submitted successively to simulated stomach and intestinal conditions. Lipid oxidation was evaluated through the measurement of oxygen consumption, the formation of hydroperoxides and malonaldehyde (MDA). With endogenous tocopherols (553 mg kg−1 oil) and without initiator no oxygen was consumed, while the presence of metmyoglobin (20 µM) induced a low oxygen consumption and formation of MDA during the gastric (73 ± 13 nmol g−1 lipids) and intestinal steps (163 ± 13 nmol g−1 lipids). Without endogenous tocopherol (<2 mg kg−1 oil) and with metmyoglobin (20 μM), amounts of hydroperoxides and MDA reached 79 ± 28 μmol g−1 lipids and 2,139 ± 78 nmol g−1 lipids, respectively, after the gastric phase and 113 ± 22 μmol g−1 lipids and 2,663 ± 38 nmol g−1 lipids during the intestinal phase. The results indicate that the composition of the food bolus, in particular the presence of lipophilic antioxidant and/or prooxidant species, is critical for the oxidative fate of emulsified lipids during digestion. Moreover, the physicochemical conditions of the digestion, rather than the constituents of simulated digestive fluids, seem to favor lipid oxidation.



Similar content being viewed by others
References
Adhikari S, Joshi R, Gopinathan C (1998) Bilirubin as an anti precipitant against copper mediated denaturation of bovine serum albumin: formation of copper–bilirubin complex. Biochim Biophys Acta 1380(1):109–114
Armand M, Borel P, Pasquier B, Dubois C, Senft M, Andre M, Peyrot J, Salducci J, Lairon D (1996) Physicochemical characteristics of emulsions during fat digestion in human, stomach and duodenum. Am J Physiol Gastrointest Liver Physiol 271(34):G172–G183
Armand M, Pasquier B, Andre M, Borel P, Senft M, Peyrot J, Salducci J, Portugal H, Jaussan V, Lairon D (1999) Digestion and absorption of 2 fat emulsions with different droplet sizes in the human digestive tract. Am J Clin Nutr 70(6):1096–1106
Awada M, Soulage CO, Meynier A, Debard C, Plaisancié P, Benoit B, Picard G, Loizon E, Chauvin MA, Estienne M, Peretty N, Guichardant M, Lagarde M, Genot C, Michalski MC (2012) Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: role of intestinal absorption of 4-HHE and reactivity in intestinal cells. J Lipid Res 53(10):2069–2080
Baynes JW (2007) Dietary ales are a risk to human health-not. Mol Nutr Food Res 51:1102–1106
Bergamo P, Fedele E, Balestrieri M, Abrescia P, Ferrara L (1998) Measurement of malondialdehyde levels in food by high-performance liquid chromatography with fluorimetric detection. J Agric Food Chem 46:2171–2176
Berton C, Genot C, Ropers MH (2011) Quantification of unadsorbed protein and surfactant emulsifiers in oil-in-water emulsions. J Colloid Interface Sci 354(2):739–748
Berton C, Ropers MH, Viau M, Genot C (2011) Contribution of the interfacial layer to the protection of emulsified lipids against oxidation. J Agric Food Chem 59(9):5052–5061
Brogard M, Troedsson E, Thuresson K, Ljusberg-Wahren H (2007) A new standardized lipolysis approach for characterization of emulsions and dispersions. J Colloid Interface Sci 308(2):500–507
Cohn JS (2002) Oxidized fat in the diet, postprandial lipaemia and cardiovascular disease. Curr Opin Lipidol 13(1):19–24
Dobarganes C, Marquez-Ruiz G (2003) Oxidized fats in foods. Curr Opin Clin Nutr Metabol Care 6(2):157–163
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128
Eymard S, Genot C (2003) A modified xylenol orange method to evaluate formation of lipid hydroperoxides during storage and processing of small pelagic fish. Eur J Lipid Sci Technol 205(9):497–501
Garrett DA, Failla ML, Sarama RJ (1999) Development of an in vitro digestion method to assess carotenoid bioavailability from meals. J Agric Food Chem 47(10):4301–4309
Genot C, Meynier A, Riaublanc A (2003) Lipid oxidation in emulsions. In: Kamal-Eldin A (ed) Lipid oxidation pathways, vol 7. AOCS, Champaign, pp 190–244
Gorelik S, Ligumsky M, Kohen R, Kanner J (2008) The stomach as a “bioreactor”: when red meat meets red wine. J Agric Food Chem 56(13):5002–5007
Grau A, Guardiola F, Boatella J, Barroeta A, Codony R (2000) Measurement of 2-thiobarbituric acid values in dark chicken meat through derivative spectrophotometry: Influence of various parameters. J Agric Food Chem 48:1155–1159
Grootveld M, Atherton MD, Sheerin AN, Hawkes J, Blake DR, Richens TE, Silwood CJL, Lynch E, Claxson AWD (1998) In vivo absorption, metabolism, and urinary excretion of a, b-unsaturated aldehydes in experimental animals—relevance to the development of cardiovascular diseases by the dietary ingestion of thermally stressed polyunsaturate-rich culinary oils. J Clin Invest 101(6):1210–1218
Herrero-Barbudo MC, Granado-Lorencio F, Blanco-Navarro I, Perz-Sacristan B, Olmedilla-Alonso B (2009) Applicability of an in vitro model to assess the bioaccessibility of vitamins a and E from fortified commercial milk. Int Dairy J 19(1):64–67
Kanazawa K, Ashida H (1998) Dietary hydroperoxides of linoleic acid decompose to aldehydes in stomach before being absorbed into the body. Biochim Et Biophys Acta-Lipids and Lipid Metabol 1393(2–3):349–361
Kanner J (2007) Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol Nutr Food Res 51:1094–1101
Kanner J, Lapidot T (2001) The stomach as a bioreactor: dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radic Biol Med 31(11):1388–1395
Kenmogne-Domguia HB, Meynier A, Viau M, Llamas G, Genot C (2012) Gastric conditions control both the evolution of the organization of protein-stabilized emulsions and the kinetic of lipolysis during in vitro digestion. Food Funct. doi:10.1039/c2fo30031a
Lorrain B, Dangles O, Genot C, Dufour C (2010) Chemical modeling of heme-induced lipid oxidation in gastric conditions and inhibition by dietary polyphenols. J Agric Food Chem 58(1):676–683
Lorrain B, Dangles O, Loonis M, Armand M, Dufour C (2012) Dietary iron-initiated lipid oxidation and its inhibition by polyphenols in gastric conditions. J Agric Food Chem 60(36):9074–9081
Mendes R, Cardoso C, Pestana C (2009) Measurement of malondialdehyde in fish: a comparison study between HPLC methods and the traditional spectrophotometric test. Food Chem 112:1038–1045
Nourooz-Zadeh J, Tajaddini-Sarmadi J, Wolff SP (1995) Measurement of hydroperoxides in edible oils using ferrous oxidation in xylenol orange assay. J Agric Food Chem 43(1):17–21
Seljeskog E, Hervig T, Mansoor MA (2006) A novel HPLC method for the measurement of thiobarbituric acid reactive substances (TBARS). A comparison with a commercially available kit. Clin Biochem 39:947–954
Staprans I, Rapp JH, Pan XM, Feingold KR (1996) Oxidized lipids in the diet are incorporated by the liver into very low density lipoprotein in rats. J Lipid Res 37:420–430
Tagliazucchi D, Verzelloni E, Conte A (2010) Effect of dietary melanoidins on lipid peroxidation during simulated gastric digestion: their possible role in the prevention of oxidative damage. J Agric Food Chem 58(4):2513–2519
Terao J, Ingemansson T, Ioku K, Yuki H, Ito Y (1995) Effects of rat bile–pancreatic juice on Fe2+ induced peroxidation of phospholipids. Biosci Biotechnol Biochem 59(1):55–58
Tesoriere L, Butera D, Gentile C, Livrea MA (2007) Bioactive components of caper (Capparis spinosa L.) from Sicily and antioxidant effects in a red meat simulated gastric digestion. J Agric Food Chem 55(21):8465–8471
Tyssandier V, Reboul E, Dumas JF, Bougteloup-Demange C, Armand M, Marcand J, Sallas M, Borel P (2003) Processing of vegetable-borne carotenoids in the human stomach and duodenum. Am J Physiol Gastrointest Liver Physiol 284(6):G913–G923
Ursini F, Zamburlini A, Cazzolato G, Maiorino M, Bon GB, Sevanian A (1998) Postprandial plasma lipid hydroperoxides: a possible link between diet and atherosclerosis. Free Radic Biol Med 25(2):250–252
Verzelloni E, Tagliazucchi D, Conte A (2010) From balsamic to healthy: traditional balsamic vinegar melanoidins inhibit lipid peroxidation during simulated gastric digestion of meat. Food Chem Toxicol 48(8–9):2097–2102
Villière A, Viau M, Bronnec I, Moreau N, Genot C (2005) Oxidative stability of bovine serum albumin and sodium caseinate-stabilized emulsions depends on metal availability. J Agric Food Chem 53(5):1514–1520
Acknowledgments
This work was supported by INRA and Région Pays de la Loire: PhD grant of H.B.K.-D., and by ANR (French National Research Agency) program “Alimentation et industries alimentaires—Food and food industry”: ANR-08-ALIA-002 AGEcaninox project. Authors participate to the COST action FA1005 Improving health properties of food by sharing our knowledge on the digestive process (INFOGEST). Lucie Ribourg is acknowledged for her help in tocopherols quantification and for measurements of oxidation during accelerated oxidation of the oil.
Author information
Authors and Affiliations
Corresponding author
Additional information
The paper was based on a poster presented during the 1st International Conference on Food Digestion (Cesena, Italy, March 19–21, 2012) organized by COST action FA1005 INFOGEST.
Rights and permissions
About this article
Cite this article
Kenmogne-Domguia, H.B., Meynier, A., Boulanger, C. et al. Lipid Oxidation in Food Emulsions Under Gastrointestinal-Simulated Conditions: the Key Role of Endogenous Tocopherols and Initiator. Food Dig. 3, 46–52 (2012). https://doi.org/10.1007/s13228-012-0026-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13228-012-0026-9