Abstract
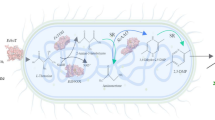
D-Pantothenic acid, as a momentous vitamin, is extensively applied to feed, medicine, cosmetics and other fields. However, there are still limitations to produce D-pantothenic acid by microbial fermentation at present. In this paper, we constructed a recombinant strain for D-pantothenic acid production by blocking the organic acid pathway, boosting pyruvate biosynthesis, relieving feedback inhibition of acetolactate synthase, improving glucose intake capacity, and modifying essential genes in the metabolic pathway. In addition, a new acetolactate isomeroreductase mutant V412A origin from Escherichia coli (EcAHAIR) encoded by ilvC was obtained to explore its substrate promiscuity. Compared with the wild type, the variant EcAHAIR-V412A has reduced steric hindrance and enhanced intermolecular forces, resulting in a high affinity for 2-acetolactate. Eventually, the fermentation production of the final strain DPAN19/trc-ilvCV412A reached 4.65 g/L, increased by 192.5% compared with strain DPA8 in shake flask cultivation and produced 62.82 g/L D-pantothenic acid in a 5 L bioreactor. The metabolic engineering strategies and enzyme modification approaches described in this paper provide a particular perspective for the bio-manufacturing of D-pantothenic acid, branched-chain amino acids and its derivates.





Similar content being viewed by others
Abbreviations
- D-PA:
-
D-pantothenic acid
- TCA:
-
Tricarboxylic acid
- D-PL:
-
D-valerolactone
- 3-AP:
-
3-Aminopropionic acid
- AHAS:
-
Acetolactate synthase
- AHAIR:
-
Acetolactate isomeroreductase
- PCR:
-
Polymerase chain reaction
- IPTG:
-
Isopropyl-beta-D-thiogalactoside
- LB:
-
Luria–Bertani
- CRISPR:
-
Clustered Regularly Interspersed Short Palindromic Repeats
- BCAAs:
-
Branched-chain amino acids
- PEP:
-
Phosphoenolpyruvate
- 2-KIV:
-
2-Ketoisovalerate
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NADH:
-
Nicotinamide adenine dinucleotide
References
Leonardi R, Jackowski S (2007) Biosynthesis of pantothenic acid and coenzyme A. EcoSal Plus 2(2). https://doi.org/10.1128/ecosalplus.3.6.3.4
Xu JS, Patassini S, Begley P, Church S, Waldvogel HJ, Faull RLM, Unwin RD, Cooper GJS (2020) Cerebral deficiency of vitamin B5 (d-pantothenic acid; pantothenate) as a potentially-reversible cause of neurodegeneration and dementia in sporadic Alzheimer’s disease. Biochem Biophys Res Commun 527(3):676–681. https://doi.org/10.1016/j.bbrc.2020.05.015
Hall JE, Hall ME (2020) Dietary balances; regulation of feeding; obesity and starvation; vitamins and minerals, in: Hall JE, Hall ME, Guyton and Hall Textbook of Medical Physiology. fourteenth ed Elsevier United Kingdom pp:877–892.
Tigu F, Zhang JL, Liu GX, Cai Z, Li Y (2018) A highly active pantothenate synthetase from Corynebacterium glutamicum enables the production of D-pantothenic acid with high productivity. Appl Microbiol Biotechnol 102:6039–6046. https://doi.org/10.1007/s00253-018-9017-2
Postaru M, Cascaval D, Galaction AI (2015) Pantothenic acid-applications, synthesis and biosynthesis. Rev Med Chir Soc Med Nat Iasi 119(3):938–943
Huang J, Huang L, Lin JP, Xu ZN, Cen PL (2010) Organic chemicals from bioprocesses in China. Adv Biochem Eng Biotechnol 122:43–71. https://doi.org/10.1007/10_2010_75
Rowicki T, Synoradzki L, Wlostowski M (2006) Calcium pantothenate, part 1: (R, S)-pantolactone technology improvement at the tonnage scale. Ind Eng Chem Res 45:1259–1265. https://doi.org/10.1021/ie050774u
Honda K, Kataoka M, Shimizu S (2002) Functional analyses and application of microbial lactonohydrolases. Biotechnol Bioprocess Eng 7:130–137. https://doi.org/10.1007/BF02932910
Bonrath W, Netscher T (2005) Catalytic processes in vitamins synthesis and production. Appl Catal A-Gen 280:55–73. https://doi.org/10.1016/j.apcata.2004.08.028
Zou SP, Zhao K, Tang H, Zhang Z, Zhang B, Liu ZQ, Zheng YG (2021) Improved production of D-pantothenic acid in Escherichia coli by integrated strain engineering and fermentation strategies. J Biotechnol 339:65–72. https://doi.org/10.1016/j.jbiotec.2021.07.014
Grzegorz K, Macko D, Mikulski D (2010) Development of biotechnological methods of biofuels production from renewable sources. Environ Protein Nat Resour 45:118–135. https://www.researchgate.net/publication/234125774
Gao H, Tuyishime P, Zhang X, Yang TW, Xu MJ, Rao ZM (2021) Engineering of microbial cells for L-valine production: challenges and opportunities. Microb Cell Fact 20:172. https://doi.org/10.1186/s12934-021-01665-5
Liu PP, Xu HT, Zhang XL (2021) Metabolic engineering of microorganisms for L-alanine production. J Ind Microbiol Biotechnol 49(2). https://doi.org/10.1093/jimb/kuab057
Wang YY, Xu JZ, Zhang WG (2019a) Metabolic engineering of l-leucine production in Escherichia coli and Corynebacterium glutamicum: a review. Crit Rev Biotechnol 39(5):633–647. https://doi.org/10.1080/07388551.2019.1577214
Zhang B, Chen L, Jin JY, Zhong N, Cai X, Zou SP, Zhou HY, Liu ZQ, Zheng YG (2021) Strengthening the (R)-pantoate pathway to produce D-pantothenic acid based on systematic metabolic analysis. Food Biosci 43:101283. https://doi.org/10.1016/j.fbio.2021.101283
Guo JX, Sun XX, Yuan YJ, Chen QT, Ou ZT, Deng ZX, Ma T, Liu TG (2023) Metabolic Engineering of Saccharomyces cerevisiae for Vitamin B5 Production. J Agric Food Chem 71(19):7408–7417. https://doi.org/10.1021/acs.jafc.3c01082
Tyagi R, Lee YT, Guddat LW, Duggleby RG (2005) Probing the mechanism of the bifunctional enzyme ketol-acid reductoisomerase by site-directed mutagenesis of the active site. FEBS J 272(2):593–602. https://doi.org/10.1111/j.1742-4658.2004.04506.x
Bastian S, Liu X, Meyerowitz JT, Snow CD, Chen MMY, Arnold FH (2011) Engineered ketol-acid reductoisomerase and alcohol dehydrogenase enable anaerobic 2-methylpropan-1-ol production at theoretical yield in Escherichia coli. Metab Eng 13(3):345–352. https://doi.org/10.1016/j.ymben.2011.02.004
Geraskina NV, Sycheva EV, Samsonov VV, Eremina NS, Hook CD, Serebrianyi VA, Stoynova NV (2019) Engineering Escherichia coli for autoinducible production of L-valine: an example of an artificial positive feedback loop in amino acid biosynthesis. PLoS ONE 14(4):e0215777. https://doi.org/10.1371/journal.pone.0215777
Dumas R, Butikofer MC, Job D, Douce R (1995) Evidence for two catalytically different magnesium-binding sites in acetohydroxy acid isomeroreductase by site-directed mutagenesis. Biochemistry 34(18):6026. https://doi.org/10.1021/bi00018a004
Wang YY, Zhang F, Xu JZ, Zhang WG, Chen XL, Liu LM (2019b) Improvement of L-Leucine production in Corynebacterium glutamicum by altering the redox flux. Int J Mol Sci 20(8):2020. https://doi.org/10.3390/ijms20082020
Hao YA, Ma Q, Liu XQ, Fan XG, Men JX, Wu HY, Jiang S, Tian DG, Xiong B, Xie XX (2020) High-yield production of L-valine in engineered Escherichia coli by a novel two-stage fermentation. Metab Eng 62:198–206. https://doi.org/10.1016/j.ymben.2020.09.007
Zou SP, Zhang Z, Zhao K, Liu ZQ, Zheng YG (2022) Metabolic engineering of Escherichia coli for improved D-pantothenic acid biosynthesis by enhancing NADPH availability. Biochem Eng J 187:108603. https://doi.org/10.1016/j.bej.2022.108603
Zhang B, Zhang XM, Wang W, Liu ZQ, Zheng YG (2019) Metabolic engineering of Escherichia coli for d-pantothenic acid production. Food Chem 294:267–275. https://doi.org/10.1016/j.foodchem.2019.05.044
Gray LR, Tompkins SC, Taylor EB (2014) Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci 71(14):2577–2604. https://doi.org/10.1007/s00018-013-1539-2
Ma YC, Ma Q, Cui Y, Du LH, Xie XX, Chen N (2019) Transcriptomic and metabolomics analyses reveal metabolic characteristics of L-leucine- and L-valine-producing Corynebacterium glutamicum mutants. Ann Microbiol 69(5):457–468. https://doi.org/10.1007/s13213-018-1431-2
Warnecke T, Gill RT (2005) Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microb Cell Factories 4(1):1–8. https://doi.org/10.1186/1475-2859-4-25
Dittrich CR, Bennett GN, San KY (2008) Characterization of the acetate-producing pathways in Escherichia coli. Biotechnol Prog 21(4):1062–1067. https://doi.org/10.1021/bp050073s
Kim SH, Schneider BL, Reitzer L (2010) Genetics and regulation of the major enzymes of alanine synthesis in Escherichia coli. J Bacteriol 192(20):5304–5311. https://doi.org/10.1128/jb.00738-10
McCloskey D, Xu SB, Sandberg TE, Brunk E, Hefner Y, Szubin R, Feist AM, Palsson BO (2018) Adaptive laboratory evolution resolves energy depletion to maintain high aromatic metabolite phenotypes in Escherichia coli strains lacking the Phosphotransferase System. Metab Eng 48:233–242. https://doi.org/10.1016/j.ymben.2018.06.005
Park JH, Kim TY, Lee KH, Lee SY (2011) Fed-batch culture of Escherichia coli for L-valine production based on in silico flux response analysis. Biotechnol Bioeng 108(4):934–946. https://doi.org/10.1002/bit.22995
Michalowski A, Siemann-Herzberg M, Takors R (2017) Escherichia coli HGT: Engineered for high glucose throughput even under slowly growing or resting conditions. Metab Eng 40:93–103. https://doi.org/10.1016/j.ymben.2017.01.005
Lunin VV, Li Y, Schrag JD, Lannuzzi P, Cygler M, Matte A (2004) Crystal structures of Escherichia coli ATP-dependent glucokinase and its complex with glucose. J Bacteriol 186(20):6915–6927. https://doi.org/10.1128/jb.186.20.6915-6927.2004
Deutscher J, Francke C, Postma PW (2006) How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev 70(4):939–1031. https://doi.org/10.1128/mmbr.00024-06
Ronneau S, Hallez R (2019) Make and break the alarmone: regulation of (p)ppGpp synthetase/hydrolase enzymes in bacteria. FEMS Microbiol Rev 43(4):389–400. https://doi.org/10.1093/femsre/fuz009
Frank RAW, Price AJ, Northrop FD, Perham RN, Luisi BF (2007) Crystal structure of the E1 component of the Escherichia coli 2-oxoglutarate dehydrogenase multienzyme complex. J Mol Biol 368(3):639–651. https://doi.org/10.1016/j.jmb.2007.01.080
Chen W, Yao J, Meng J, Han WJ, Tao Y, Chen YH, Guo YX, Shi GZ, He Y, Jin JM, Tang SY (2019) Promiscuous enzymatic activity-aided multiple-pathway network design for metabolic flux rearrangement in hydroxytyrosol biosynthesis. Nat Commun 10(1):960. https://doi.org/10.1038/s41467-019-08781-2
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFA0901400).
Funding
This work was supported by the National Key Research and Development Program of China (2018YFA0901400).
Author information
Authors and Affiliations
Contributions
BZ: writing-original draft, writing-review, editing. YQZ: designed research, writing-review, data curation. ZLH: investigation, analyzed data. YYX: investigation, methodology. MNT: formal analysis. JPZ: visualization. ZQL: project administration, funding acquisition, supervision, conceptualization. YGZ: project administration, funding acquisition, supervision, conceptualization, funding acquisition, supervision.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, B., Zhang, YQ., He, ZL. et al. Engineered E. coli for D-pantothenic acid production with an acetolactate isomeroreductase mutant. 3 Biotech 14, 117 (2024). https://doi.org/10.1007/s13205-024-03931-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-024-03931-w